Evolución de sunitinib en 1ª línea en CCRm: Esquemas alternativos - page 12
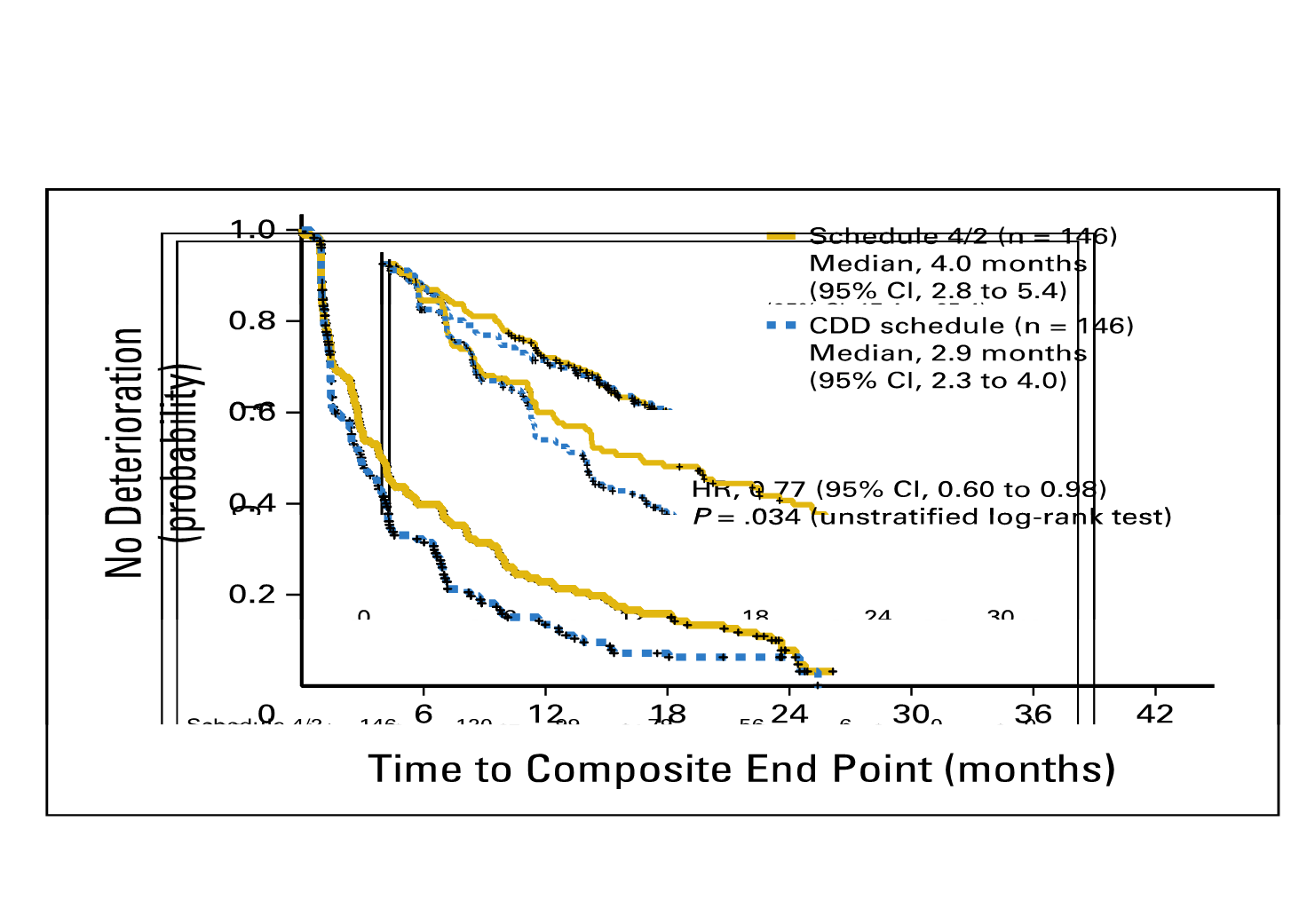
The Renal EFFECT Trial:
Resultados
Sunitinib 50 mg/d
Schedule 4/2
(N=146)
Sunitinib 37.5 mg/d
CDD schedule
(N=143)
†
Adverse Event, n (%)
All grades
Grade 3/4
All grades
Grade 3/4
Fatigue
95 (65)
13 (9)
101 (71)
16 (11)
Nausea
92 (63)
4 (3)
77 (54)
9 (6)
Diarrhea
86 (59)
9 (6)
98 (69)
8 (6)
Dysgeusia
55 (38)
0
49 (34)
0
Vomiting
49 (34)
4 (3)
49 (34)
9 (6)
Hypertension
48 (33)
12 (8)
42 (29)
12 (8)
Decreased appetite
46 (32)
2 (1)
59 (41)
7 (5)
Hand-foot syndrome
46 (32)
14 (10)
36 (25)
15 (10)
Mucosal inflammation
41 (28)
3 (2)
36 (25)
3 (2)
Constipation
40 (27)
1 (<1)
40 (28)
2 (1)
Dyspepsia
37 (25)
2 (1)
36 (25)
0
Rash
36 (25)
2 (1)
44 (31)
2 (1)
1...,2,3,4,5,6,7,8,9,10,11
13,14,15,16,17,18,19,20,21,22,...43


