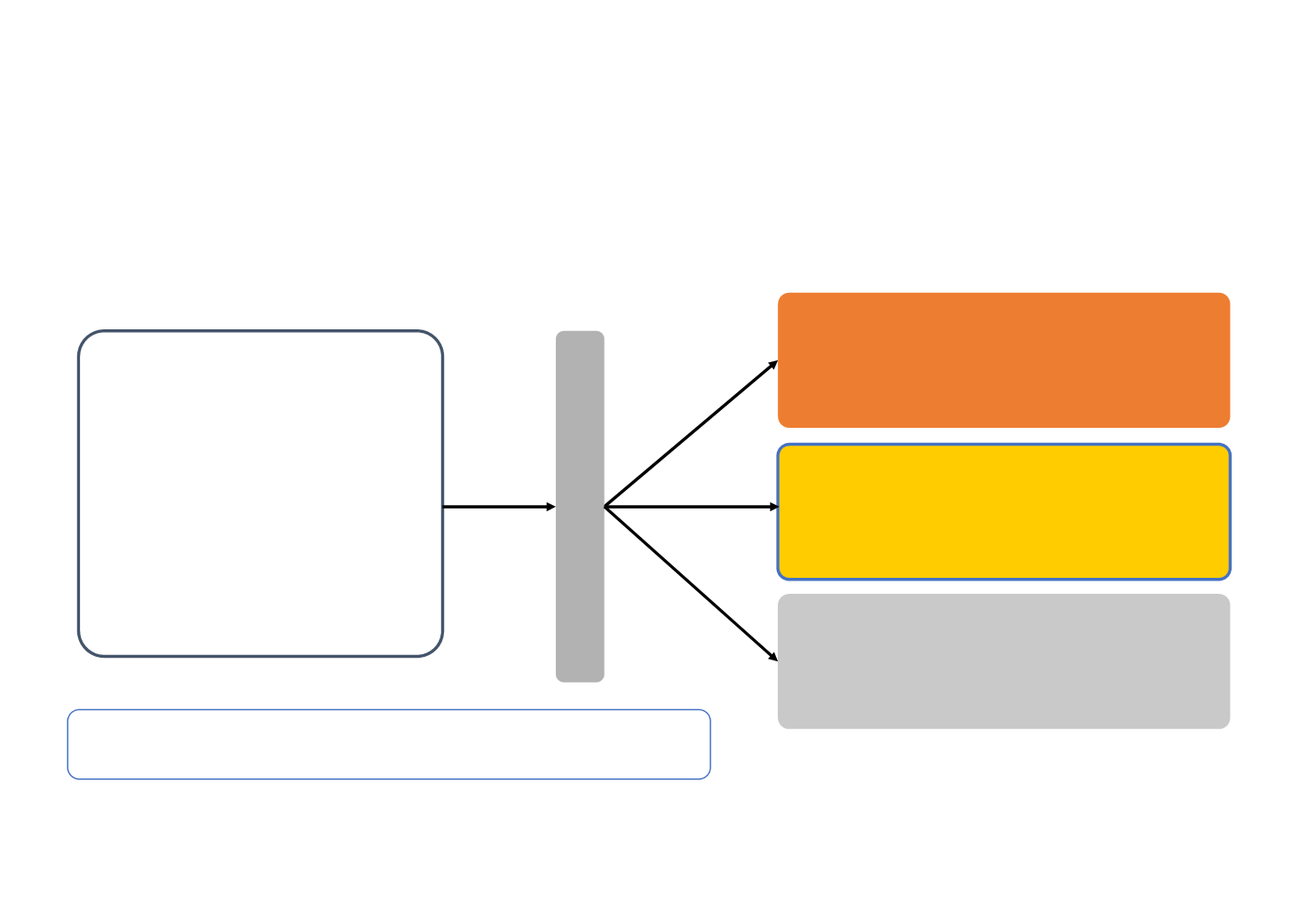
RAINBOW study: modified sunitinib scheduling in patients
ith RCC
w m
Retrospective observational study of mRCC patients administered sunitinib on a 2/1 schedule*
G A ( 208)
roup n=
Sunitinib
50 mg/day*
Switched from schedule
4/2 to 2/1 due to TEAE
Objective
B
Group B (n=41)
Sunitinib
50 mg/day*
Evaluate efficacy and safety of
2/1 vs 4/2 schedule
N=276
A
S
E
L
Schedule 2/1
ab initio
due to poor clinical
condition
Eligibility criteria
Patients with advanced RCC
I
N
E
Group C (n=27)
Sunitinib
50 mg/day*
Schedule 4/2 (control)
S f t d i t
I id
f d
t
a e y en po n
: nc ence o a verse even s
Efficacy endpoint:
PFS and treatment duration
*Dose reductions were possible
TEAE, treatment-emergent adverse events
Bracarda
et al.
ASCO GU 2014


