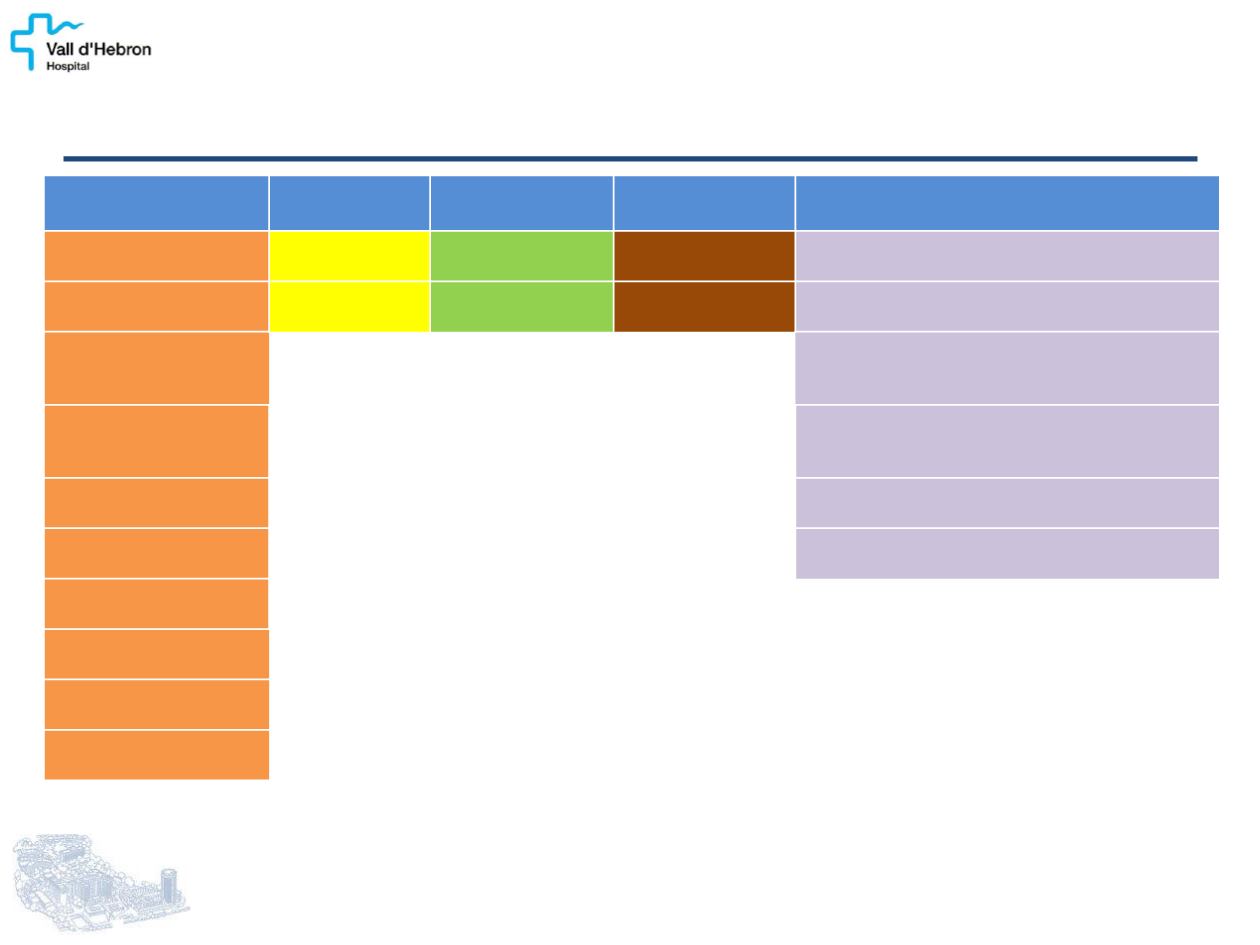
MKIs of
Angiogenesis
BRAF
Inhibitors
mTOR
Inhibitors
MEK Inhibitors
Other
(MoA)
Sorafenib
[Nexavar]
Vemurafenib
[Zelboraf]
Temsirolimus
[Toricel]
Selumetinib
[AZD6244]
Lenalidomide
(Inhibitor of angiogenesis)
Axitinib
[AG-013736]
Dabrafenib
[GSK2118436]
Everolimus
[Afinitor]
Trametinib
[GSK1120212]
Tanespimycin
[17-AAG]
(Heat shock protein 90 [HSP90] inhibitor)
Pazopanib
[GW786034,
Votrient]
VEGF trap
[Aflibercept]
(Inhibitor of angiogenesis)
Motesanib
[AMG 706]
Fosbretabulin tromethamine
[CA4P]
(Microtubule destabilizing agent, vascular-
targeting agent)
Sunitinib
[SU011248, Sutent]
Bortezomib
[Velcade]
(Proteasome inhibitor)
Vandetanib
[ZD6474, Caprelsa]
Panobinostat
[LBH589]
(Histone deacetylase inhibitor)
Lenvatinib
[E7080]
Gefitinib
[ZD1839, Iressa]
Cabozantinib
[XL-184, Cometriq]
Cediranib
[AZD2171]
Summary of Agents Being Investigated
in Thyroid Cancer
•
Two TKIs are FDA/EMA-approved for
progressive, metastatic MTC
–
Vandetanib
–
Cabozantinib
•
Sorafenib is FDA/EMA-approved for locally recurrent or metastatic,
progressive, DTC that is RAI-refractory
•
Lenvatinib is FDA/EMA approved for locally advanced or metastatic,
progressive, DTC that is RAI-refractory
•
Vandetanib Phase 3 trial just reported negative results


