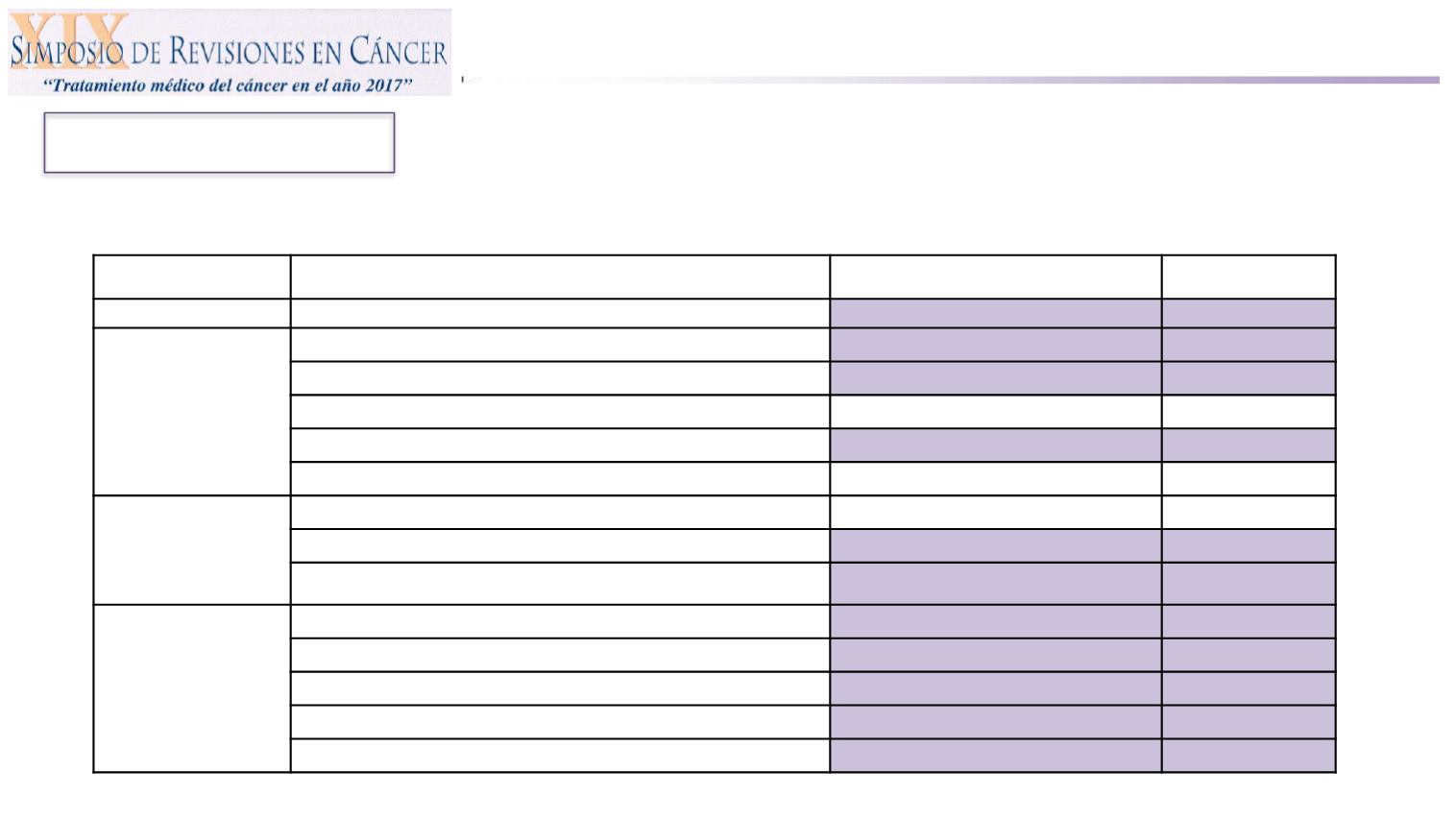
Motzer RJ, et al. N Engl J Med 2013;369(8):722.
COMPARZ. Fase III
CALIDAD DE VIDA.
PAZOPANIB: ENSAYOS CLÍNICOS.
Instrument
Domain Description
Treatment difference : mean change
from baseline
2
P -value
FACIT-F
Fatigue/Total score
2.32
<0.001
FKSI-19
Kidney Symptom Index/Total score
1.41
0.018
Physical
0.78
0.027
Emotional
0.05
0.409
Treatment Side Effects
0.31
0.033
Functional Well Being
0.31
0.098
Cancer Treatment
Satisfaction
Questionnaire
(CTSQ)
Expectations of Therapy
1.41
0.284
Feelings about Side Effects
8.50
<0.001
Satisfaction with Therapy
3.21
<0.001
SupplementaryQualit
y of Life
Questionnaire
(SQLQ)
Worst mouth/throat soreness
0.505
<0.0001
Worst foot soreness
0.204
0.0016
Worst hand soreness
0.267
0.0008
Limitations due to mouth/throat soreness
0.94
<0.001
Limitations due to foot soreness
0.65
0.014


