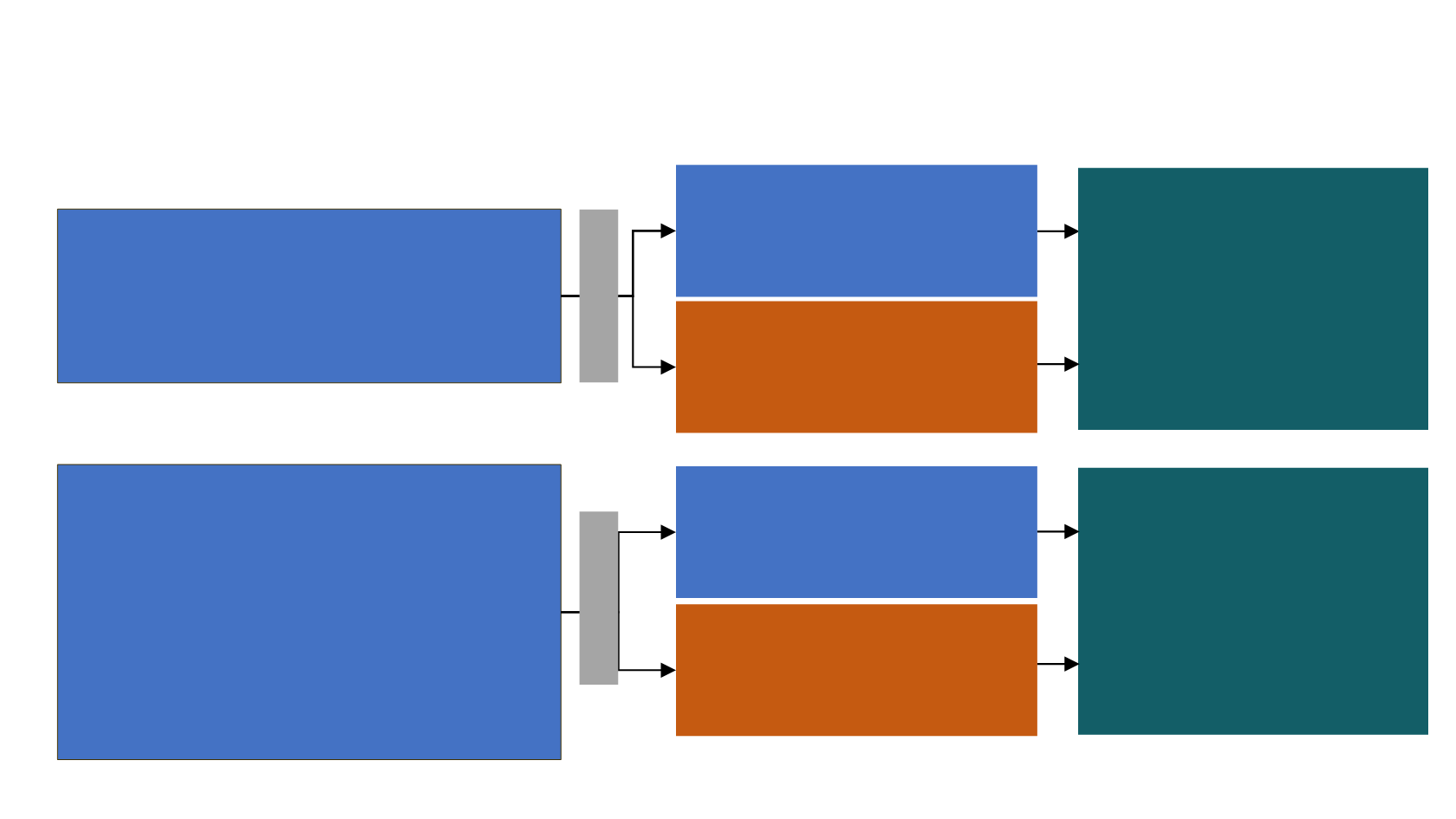
Checkmate 017 and 057
:
STUDY DESIGNS
Docetaxel
75 mg/m
2
IV Q3W
until PD or unacceptable toxicity
(n=290)
Docetaxel
75 mg/m
2
IV Q3W
until PD or unacceptable toxicity
(n=137)
Nivolumab
3 mg/kg IV Q2W
until PD or unacceptable toxicity
(n=292)
Key eligibility criteria
•
Stage IIIB/IV
non-SQ NSCLC
•
ECOG PS 0–1
•
One prior platinum-based chemotherapy
•
Pretreatment (archival or fresh) tumor
samples required for PD-L1 analysis
•
Prior maintenance therapy allowed
•
Prior TKI therapy allowed for known
ALK
translocation or
EGFR
mutation
Nivolumab
3 mg/kg IV Q2W
until PD or unacceptable toxicity
(n=135)
Endpoints
•
Primary
‒ OS
•
Additional
‒ ORR
‒ PFS
‒ Efficacy by tumor PD-L1 expression
‒ Safety
‒ Quality of life (LCSS)
Checkmate 017
(NCT01642004;
N=272)
Checkmate 057
(NCT01673867
; N=582)
Key eligibility criteria
•
Stage IIIB/IV SQ NSCLC
•
ECOG PS 0–1
•
One prior platinum-based chemotherapy
•
Pretreatment (archival or fresh) tumor
samples required for PD-L1 analysis
Endpoints
•
Primary
‒ OS
•
Additional
‒ ORR
‒ PFS
‒ Efficacy by tumor PD-L1 expression
‒ Safety
‒ Quality of life (LCSS)
Randomize 1:1
Randomize 1:1
Borghaei H et al. Poster presentation at ASCO 2016. 9025.


