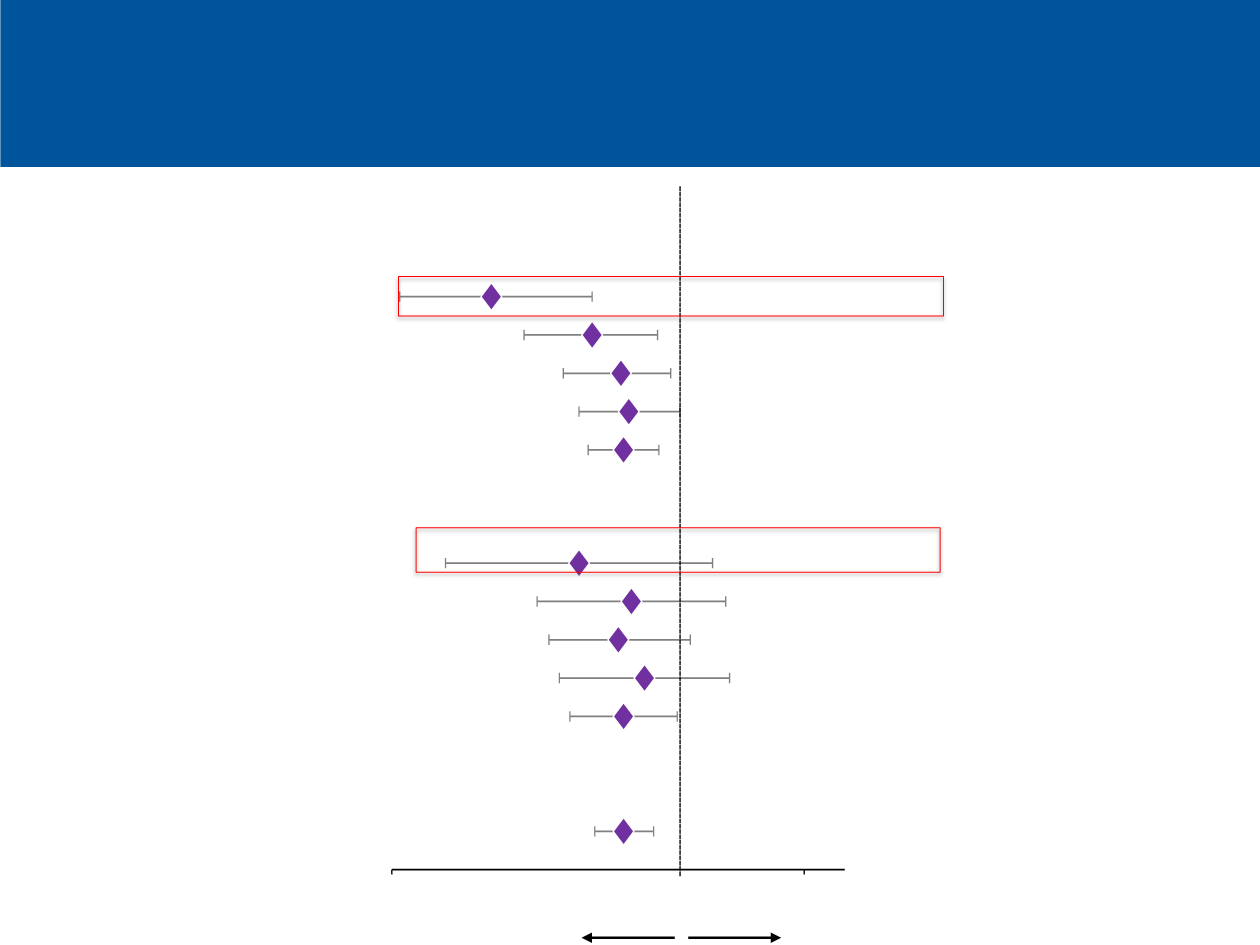
Gadgeel et al., WCLC 2016
0.35
n (%)
Subgroup
96 (15%)
Nonsquamous
Median OS, mo
Atezolizumab
Docetaxel
22.5
8.7
HR
a
TC3 or IC3
TC2/3 or IC2/3
TC1/2/3 or IC1/2/3
TC0 and IC0
All nonsquamous
Squamous
TC3 or IC3
TC2/3 or IC2/3
TC1/2/3 or IC1/2/3
TC0 and IC0
All squamous
ITT
628 (100%)
188 (30%)
3 3 (53%)
290 (46%)
41 (18%)
222 (100%)
77 (35%)
1 0 (59%)
89 (40%)
0.61
0.72
0.75
0.73
0.57
0.76
0.71
0.82
0.73
18.7
11.3
17.6
11.3
14.0
11.2
15.6
11.2
17.5
10.4
11.6
9.9
9.7
7.6
8.7
8.9
7.1
7.7
13.8
9.6
0.73
850
0.2
2
0.2 1 2
In favor of docetaxel
Hazard Ratio
In favor of atezolizumab
Histology by PD-L1 status: Overall survival


