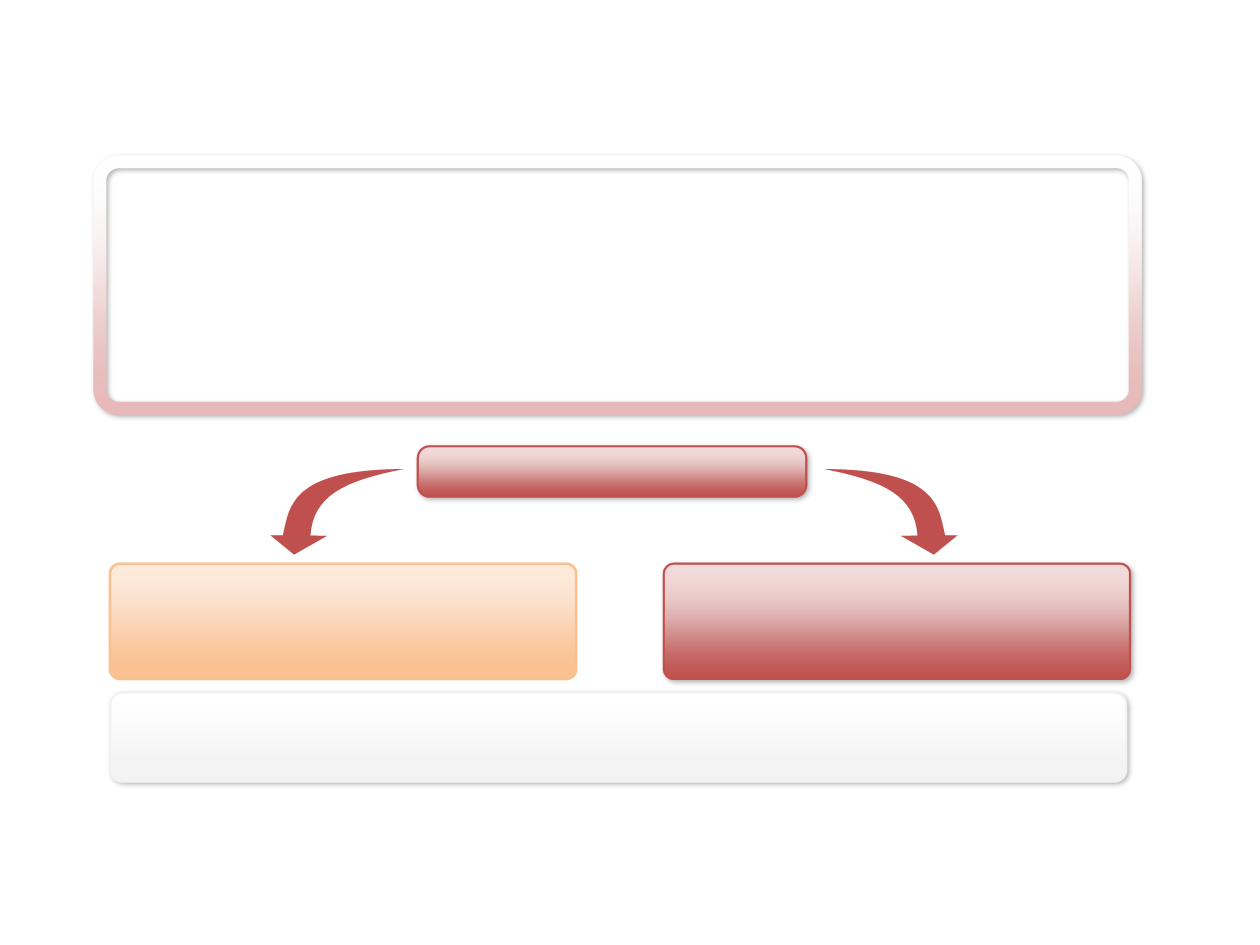
LUME-Lung 1 Pivotal Phase III Trial
Reck
et al
. Lancet Oncol 2014;15:143-55.
Patients:
•
Stage IIIB/IV
or recurrent NSCLC
•
Relapse or failed one previous first-line chemotherapy regimen
•
Eastern Cooperative Oncology Group (
ECOG
) performance status of
0 or 1
•
No active brain metastases
•
No prior treatment with docetaxel or VEGFR inhibitors with the exception of bevacizumab
Randomisation
1:1
Docetaxel
75 mg/m
2
every 3 weeks, IV
+ Nintedanib
200 mg twice daily, oral
N = 655
Docetaxel
75 mg/m
2
every 3 weeks, IV
+
Placebo
twice daily, oral
N = 659
Primary endpoint:
PFS
by independent central review
Key secondary endpoint:
OS pre-planned analyses of adenocarcinoma
and ITT
N = 1314
Stratification:
ECOG PS (0 vs. 1)
Prior bevacizumab (yes vs. no)
Histology (squamous vs. non-squamous)
Brain metastases (yes vs. no)
Regions:
Europe/Asia/South Africa
Accrual:
23 Dec 2008 to 09 Feb 2011


