Overall Survival (OS)
Randomized Phase 3 Study of Trabectedin vs Dacarbazine
(ET743-SAR-3007):
Study Design and Status at Interim Analysis
Randomization
Dexamethasone 20 mg IV pre-medication
Dacarbazine 1g/m
2
20-120 min q3wks
(N=173*)
2:1
Trabectedin 1.5 mg/m²
24h q3wks
(N=345*)
Stratification:
Prior lines chemotherapy (1 vs 2+)
ECOG PS (0 vs 1)
Sarcoma subtype (LPS vs LMS)
Key Criteria:
Histologically proven LPS or LMS
Previous therapy with an anthracycline containing regimen
and ≥ 1 additional cytotoxic chemotherapy regimen
Adequate bone marrow, renal and liver function
N=518
*
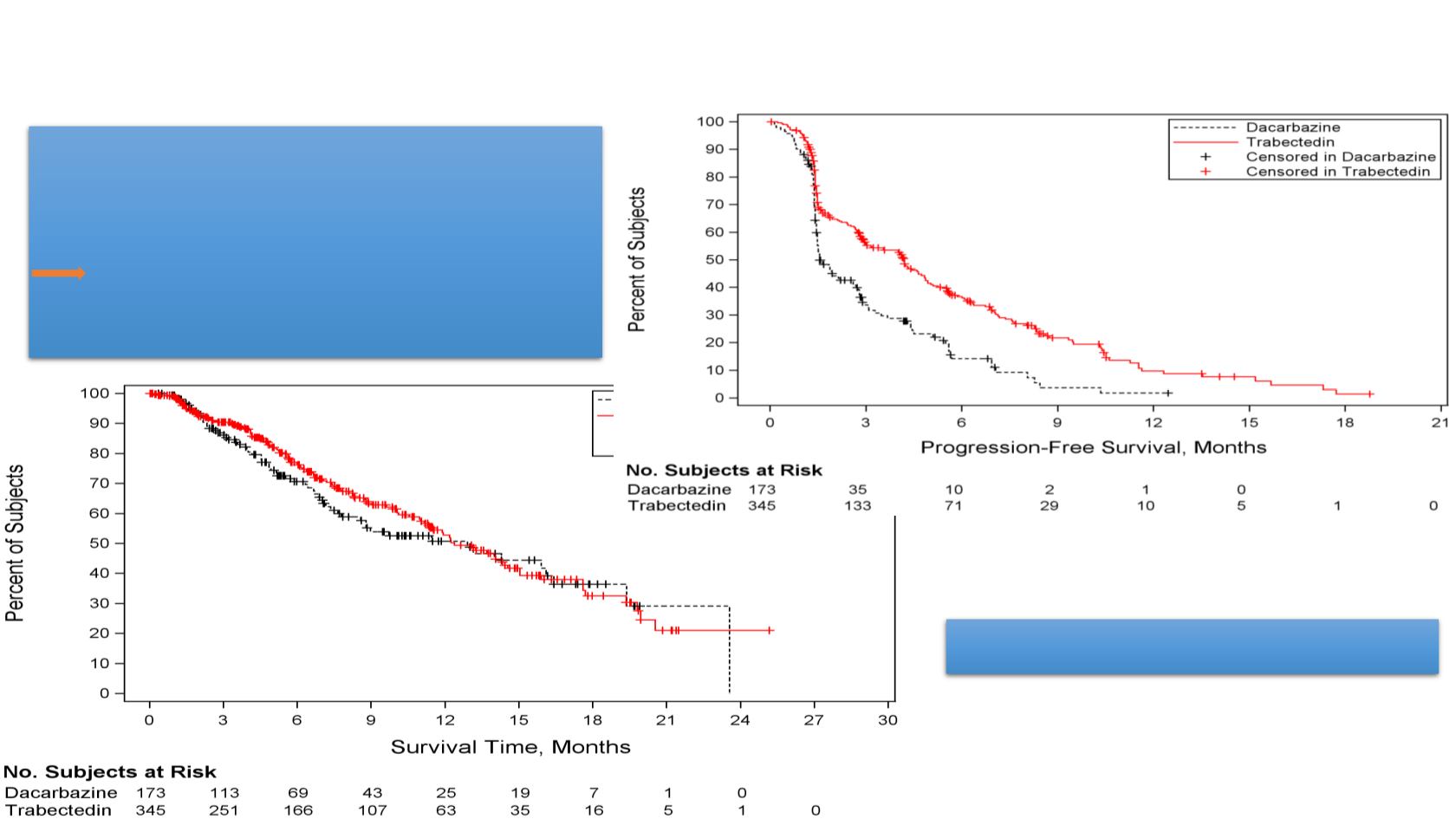
PFS events: 329 (63.5% of 518 patients)
mPFS Tr bectedin: 4.2 onths
mPFS Dacarbazine: 1.5 months
HR (95% CI) = 0.55 (0.436,0.696)
p<0.0001
OS events:189 (
64% subjects censored
)
mOS Trabectedin: 12.4 months
mOS Dacarbazine: 12.9 months
HR (95% CI)=0.87 (0.644, 1.181)
p=0.3741


