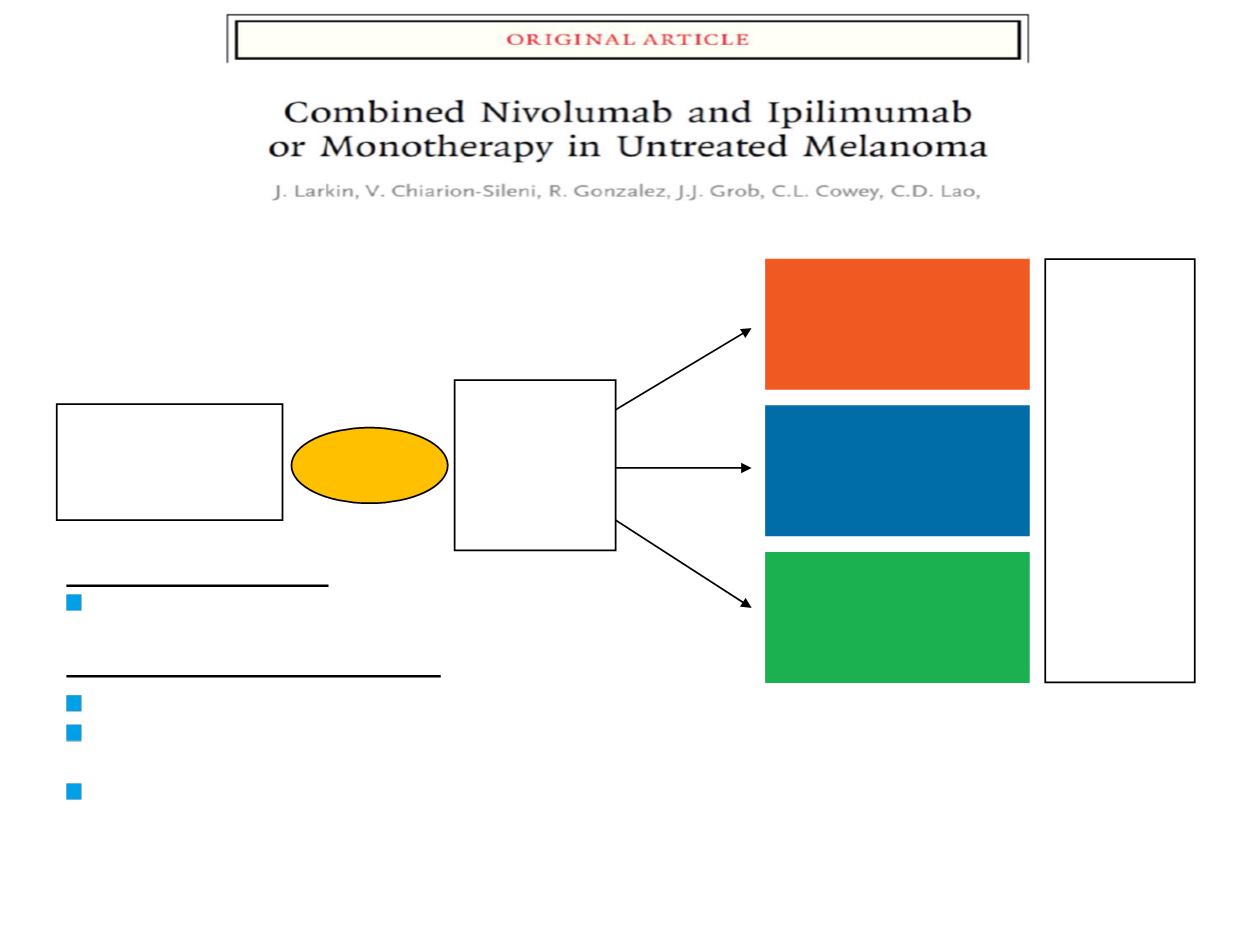
Randomized, double-blind, phase III study
to compare NIVO+IPI or NIVO alone to IPI alone
Unresectable or
Metatastic Melanoma
•
Previously untreated
•
945 patients
Treat until
progression**
or
unacceptable
toxicity
NIVO 3 mg/kg Q2W +
IPI-matched placebo
NIVO 1 mg/kg +
IPI 3 mg/kg Q3W
for 4 doses then
NIVO 3 mg/kg Q2W
IPI 3 mg/kg Q3W
for 4 doses +
NIVO-matched placebo
Randomize
1:1:1
Stratify by:
•
PD-L1
expression*
•
BRAF status
•
AJCC M stage
N=314
N=316
N=315
Co-primary endpoints:
PFS and OS (intent-to-treat population)
Secondary and other endpoints:
ORR by RECIST v1.1
Predefined tumour PD-L1 expression level as a predictive
biomarker of efficacy
Safety profile (in patients who received ≥1 dose of study drug)
Larkin et al.
N Engl J Med
2015; 373:23-34. Larkin et al. ESMO 2015; Wolchok et al ASCO 2016.
≈30% BRAF mutados, n=298


