INOVATYON DESIGN
(
IN
ternational
OVA
rian cancer patients
T
rial with
YON
delis)
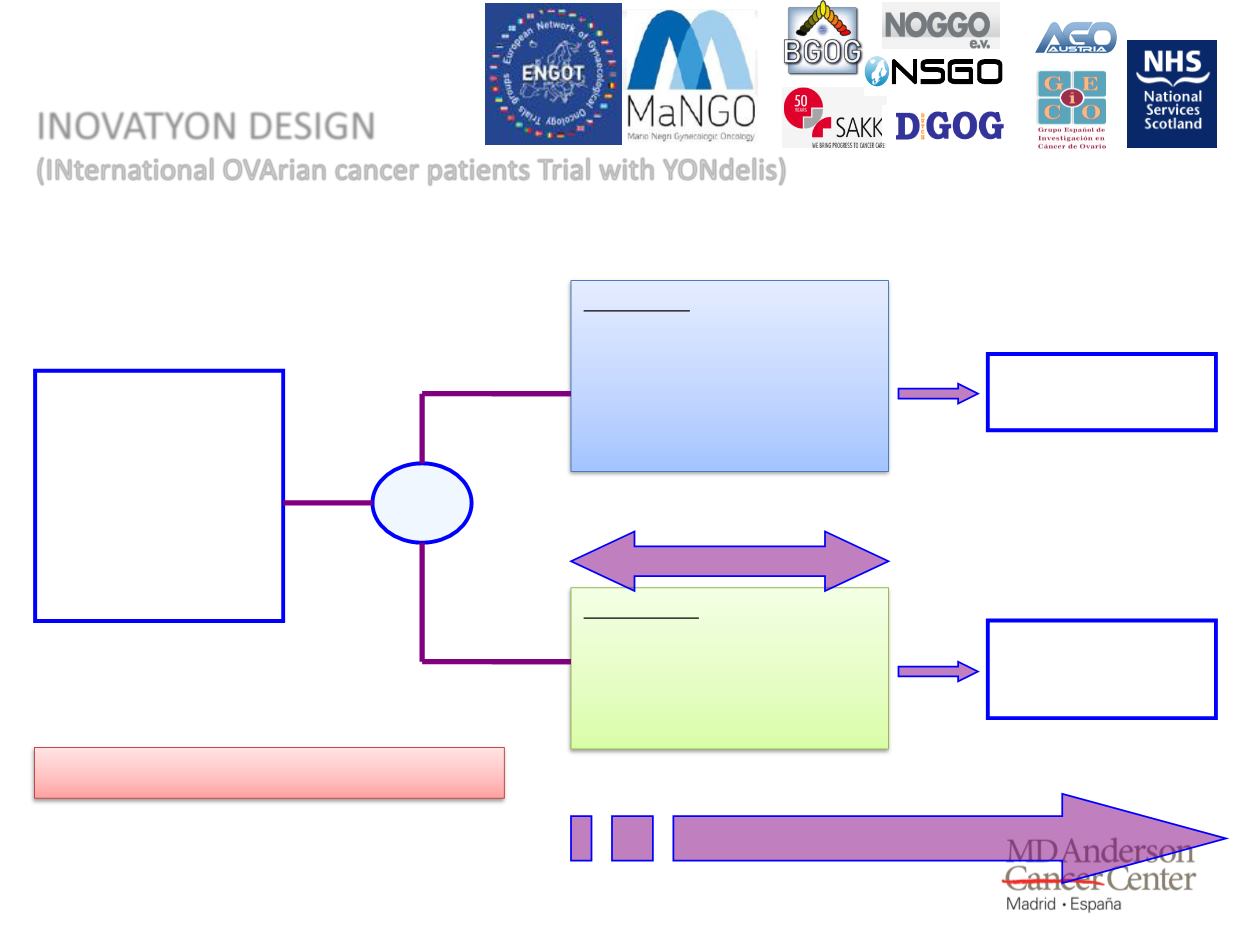
• A multicenter, randomized (1:1) phase III study
Relapsed ovarian
cancer with a
progression-free
interval of 6-12
months after the
end of 1st or 2nd
line platinum-
based therapy
Group A:
PLD 30 mg/m² 1 hour
i.v. infusion +
Carboplatin AUC 5
30-60 min i.v. on Day 1
q4 wks
At PD, subsequent
platinum rechallenge is
mandatory unless
refused by the patient
At PD, subsequent
therapy at investigator
discretion
Group B*:
PLD 30 mg/m
2
1 hour
i.v. infusion + YONDELIS
1.1 mg/m
2
3-hour i.v.
q3 wks
Tumor evaluation at week 12-24**
R
(1:1)
Up to 6 cycles or PD
OS is primary end-point


