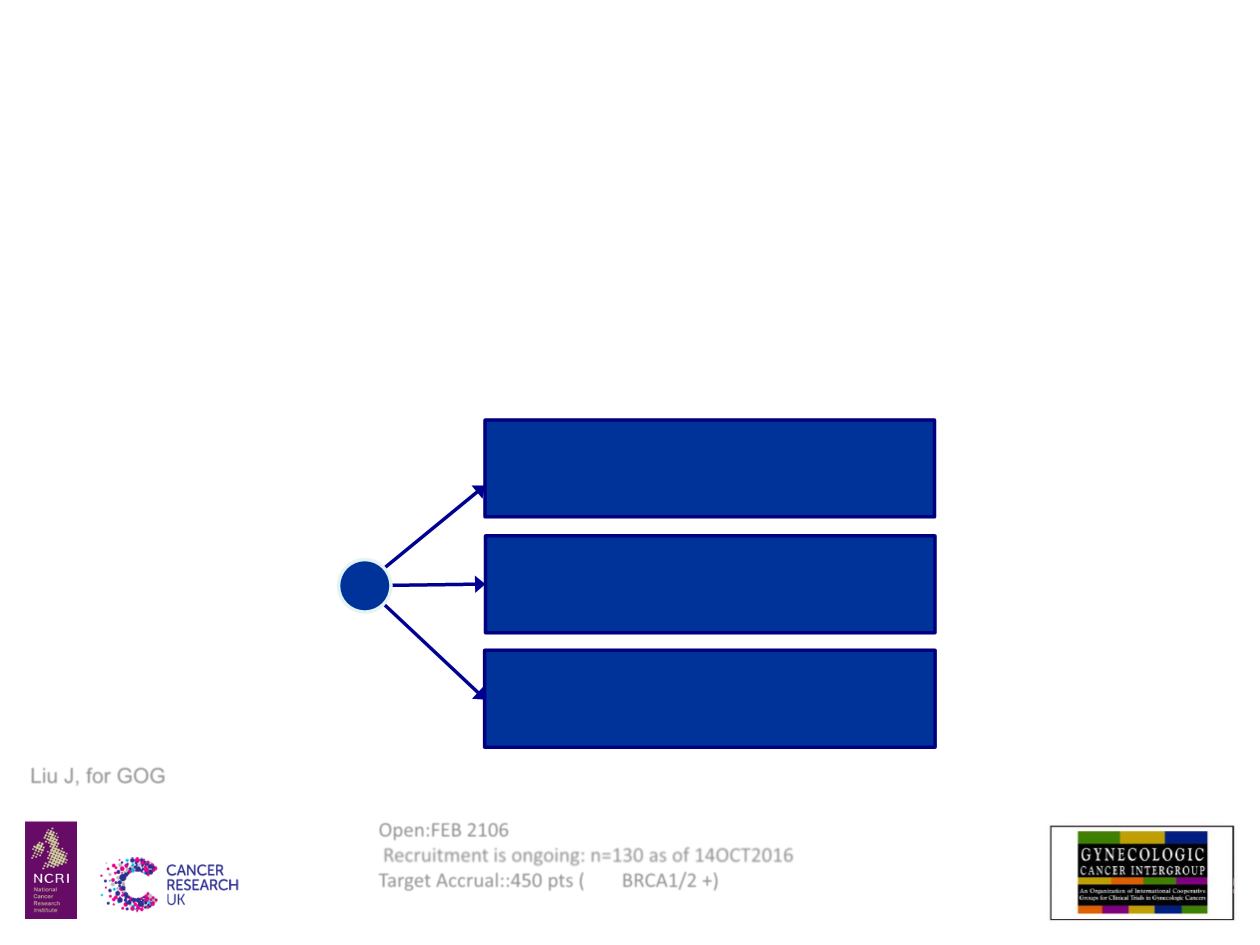
NRG-GY004: Platinum-Sensitive
• Recurrent HGSC with PFI >6 months (following most recent platinum)
• No more than 3 prior treatment regimens (including primary therapy)
• RECIST measurable or evaluable disease with accessible tumor
• No prior PARPi therapy, prior bevacizumab permitted
• Stratify for BRCA status, number of prior treatment regimens
• Primary endpoint: PFS 85% Power with HR 0.625
Cediranib 30 mg QD
Olaparib 200 mg BID
Platinum-based combo* (IV)
R
*Carboplatin + gemcitabine or paclitaxel or PLD
Olaparib 300 mg BID
Open:FEB 2106
Recruitment is ongoing: n=130 as of 14OCT2016
Target Accrual::450 pts (135 BRCA1/2 +)
Liu J, for GOG


