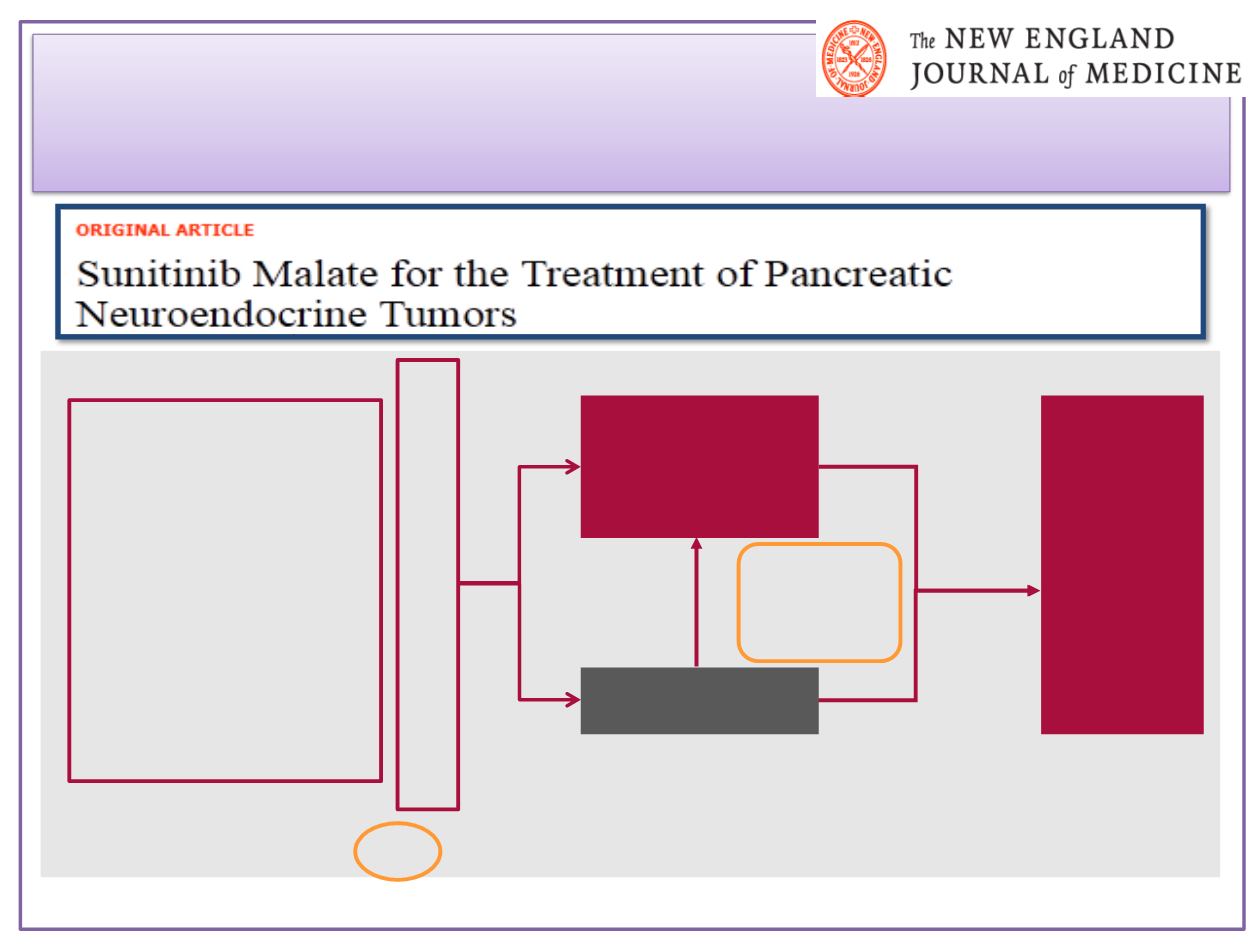
Sunitinib en
p
TNEs
G1/G2
Double blind
Primary endpoint: PFS
n=340 (planned)
R
A
N
D
O
M
I
S
A
T
I
O
N
1:1
Placebo*
(n=85)
Sunitinib
37.5 mg/day
orally, CDD*
(n=86)
Eligibility criteria
•
Well-differentiated,
malignant pancreatic
endocrine tumour
•
Disease progression
in past 12 months
•
Not amenable to
treatment with
curative intent
Stratified by region
(TBC)
•
Europe, Asia,
Americas, Australia
Study design
Study
closure
†
Open-
label
sunitinib
on
extension
study
n=171 (recruited)
Cross-over
at disease
Progression
(69%)
Raymond E, et al. N Eng J Med 2011


