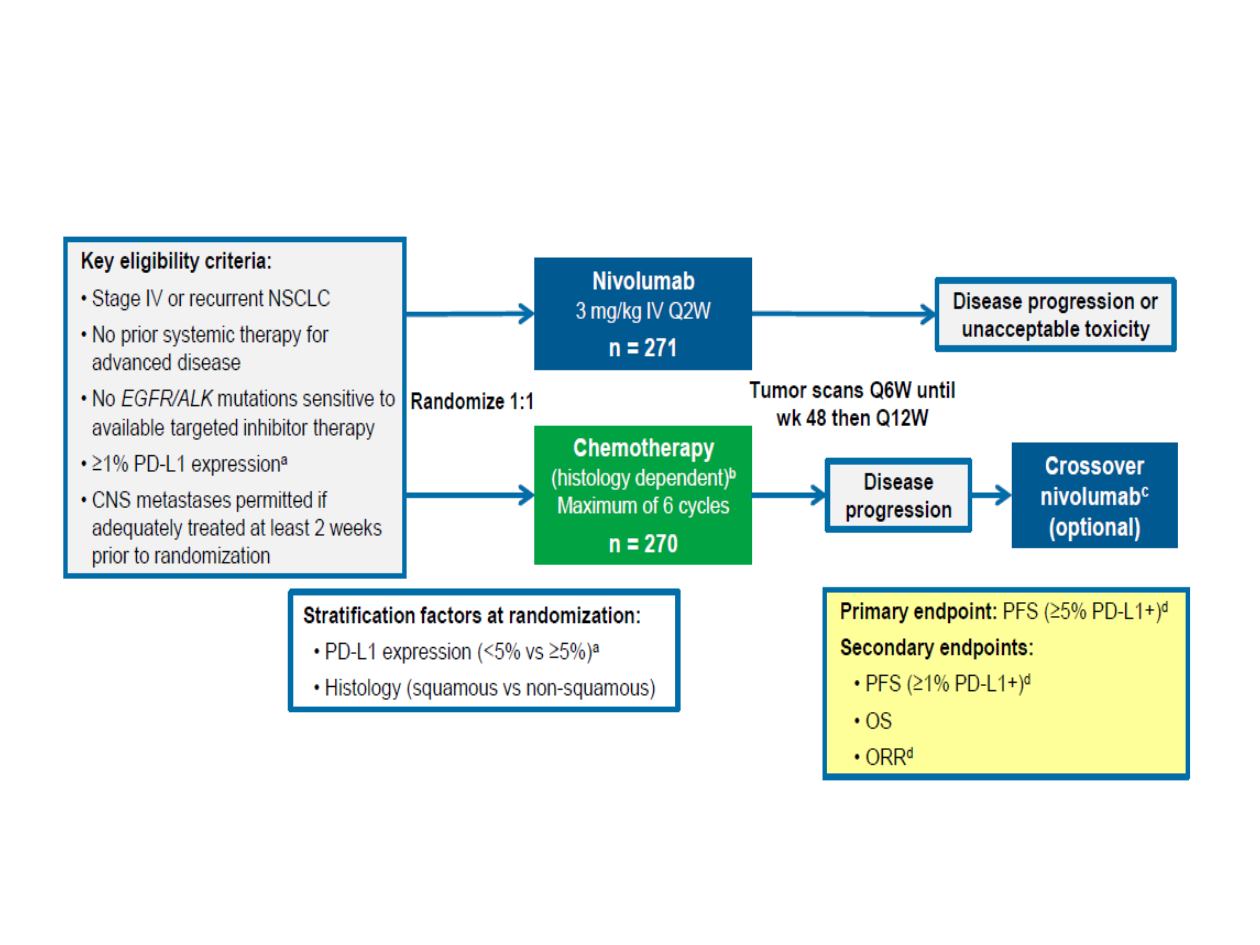
1L NSCLC: CheckMate 026
Socinski M, et al. ESMO 2016
Nivolumab
3 mg/kg IV Q2W
n = 271
Randomize
1:1
Key eligibility criteria:
•
Stage IV or recurrent NSCLC
•
No prior systemic therapy for
advanced disease
•
No
EGFR/ALK
mutations
sensitive to available targeted
inhibitor therapy
•
≥1% PD-L1 expression
a
•
CNS metastases permitted if
adequately treated at least 2
weeks prior to randomization
Chemotherapy
(histology dependent)
b
Maximum of 6 cycles
n = 270
Disease progression or
unacceptable toxicity
Disease
progression
Crossover
nivolumab
c
(optional)
Tumor scans Q6W
until wk 48 then Q12W


