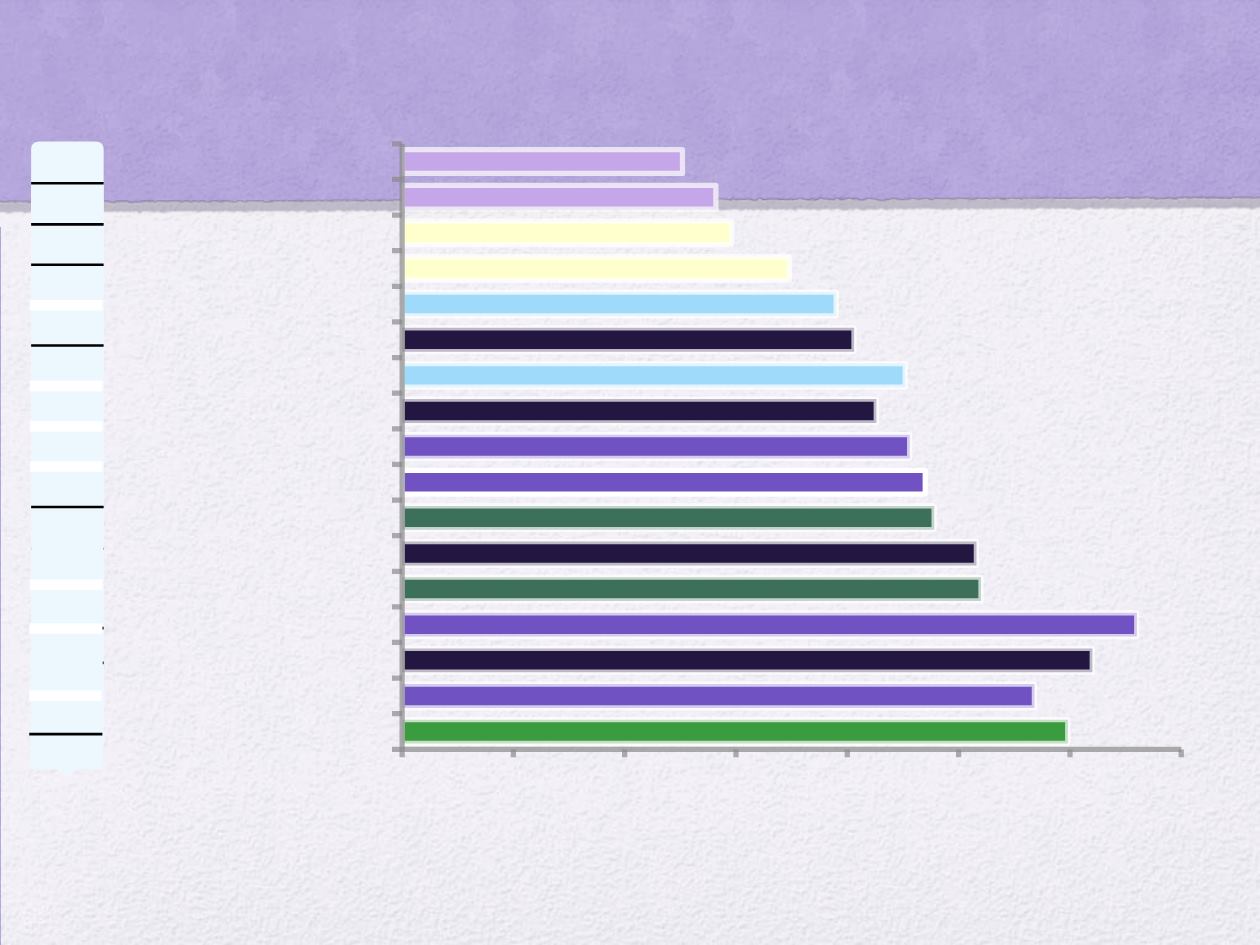
SUPERVIVENCIA EN CCRm
*
KRAS
wild type tumors; **Extended
RAS
wild type population.
1. Saltz.
N Engl J Med
. 2000; 2. Douilliard.
Lancet.
2000; 3. Goldberg.
J Clin Oncol.
2004; 4. Hurwitz.
N Engl J Med.
2004; 5. Falcone.
J Clin Oncol.
2007;
6. Saltz.
J Clin Oncol.
2008; 6. 7. Bokemeyer.
Ann Oncol.
2011; 8. Van Cutsem.
J Clin Oncol
. 2011; 9. Douilliard. ASCO 2011. Abstract 3510;
10. Heinemann. ASCO 2013. Abstract LBA3506; 11. Douillard.
New Engl J Med.
2013; 12. Stintzing. ESMO 2013. Abstract LBA17; 13. Falcone. ASCO 2013.
Abstract 3505; 14. Ciardiello. ASCO 2014. Abstract 3506; 15. Venook. ASCO 2014. Abstract LBA3.
29 – 29.9*
28.4**
30.9
26**
25.0* – 25.6**
23.9*
23.5*
21,3
22,6
20,3
19,5
17,4
14,8
14,1
12,6
0
5
10
15
20
25
30
35
Overall Survival (months)
5-FU/LV bolus
1
5-FU/LV infusion
2
IFL
1
LVFU2/irinotecan
2
FOLFOX
3
IFL + bevacizumab
4
FOLFOX/FOLFIRI
5
XELOX/FOLFOX + bevacizumab
6
FOLFOX + cetuximab
7
FOLFIRI + cetuximab
8
FOLFOX + panitumumab
9
FOLFIRI + bevacizumab
10
2013
2000
2012
2011
2011
2008
2007
2004
2004
2000
2000
2000
2011
2013
2014
FOLFOX/FOLFIRI + cetuximab or
bevacizumab
15
22.8*
FOLFOXIRI + bevacizumab
13
FOLFIRI + cetuximab
12
FOLFOX + panitumumab
11
FOLFIRI + cetuximab
14
2014
28.7*- 33.1**
Note: Informal comparison as these are not head-to-head clinical trials.


