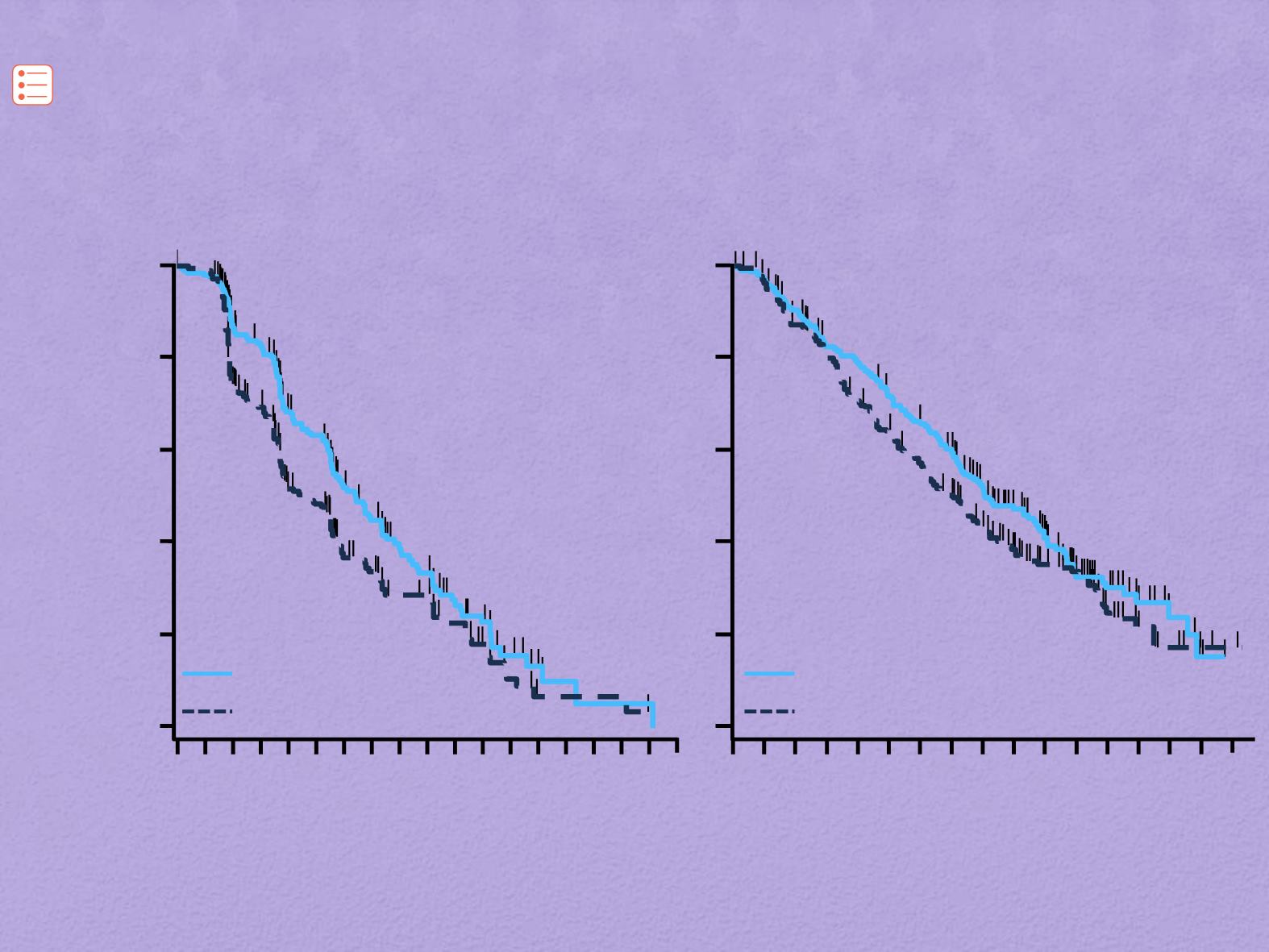
Panitumumab+ FOLFIRI en segunda linea 20050181
study
RAS
analysis
Peeters M et al. J Clin Oncol 2014;32(Suppl): Abstract 3568;
Peeters M et al. Ann Oncol 2014;25(Suppl 4):iv187–iv188 (poster 548P).
CI, confidence intervals; HR, hazard ratio; WT, wild type
100
80
60
40
20
0
0 2 4 6 8 10 12
24 26 28 30 32
14 16 18 20 22
Months
Panitumumab + FOLFIRI
FOLFIRI alone
208
213
198
203
184
181
165
164
158
144
141
129
128
113
26
20
17
12
0
1
114
98
90
77
72
61
56
46
37
38
8
5
100
80
60
40
20
0
0 1 2 3 4 5 6
12 13 14 15
18
7 8 9 10 11
Months
Proportion event-free (%)
HR: 0.70 (95% CI: 0.54, 0.91)
Log-rank p-value=0.007
208
213
Panitumumab
+ FOLFIRI
FOLFIRI alone
6.4 (5.5, 7.4)
4.6 (3.7, 5.6)
120/208 (58)
139/213 (65)
Median (95% CI),
months
Events
n/N (%)
Patients at risk:
Panitumumab
+ FOLFIRI
FOLFIRI alone
16 17
193
204
152
141
141
126
105
80
95
74
69
48
9
6
4
2
2
2
1
2
0
0
57
42
43
31
37
30
23
20
18
13
1
2
1
0
HR: 0.81 (95% CI: 0.63, 1.03)
Log-rank p-value=0.08
Panitumumab
+ FOLFIRI
FOLFIRI alone
16.2 (14.5, 19.7)
13.9 (11.9, 16.0)
130/208 (63)
143/213 (67)
Median (95% CI),
months
Events
n/N (%)
3
3
OS
PFS
Panitumumab + FOLFIRI
FOLFIRI alone
WT
RAS
WT
RAS


