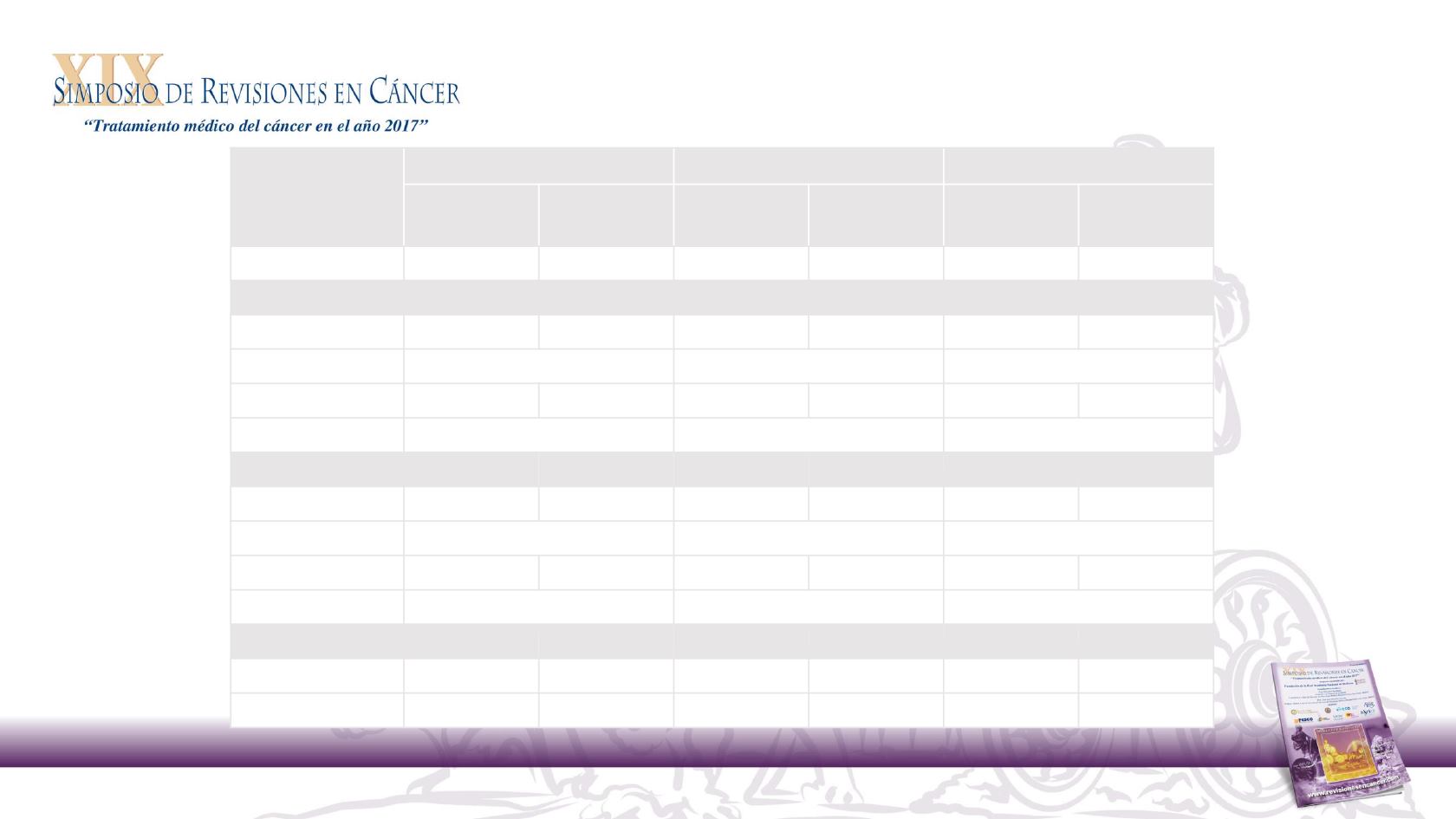
Panitumumab outcome by tumour location analysis
Efficacy outcomes by tumour location in patients with
WT
RAS
disease
•
Boeckx N, et al. Ann Oncol 2016;27(Suppl 6):abstract 89P (and poster).
•
†
Updated values will be included in the full publication.
WT
RAS
PRIME
PEAK
Study 181
Pmab +
FOLFOX
FOLFOX
Pmab +
FOLFOX
Bev + FOLFOX
Pmab +
FOLFIRI
FOLFIRI
Pts, n (left/right)
169/39
159/49
53/22
54/14
150/31
148/39
Median OS, months
Left
30.3
23.6
43.4
32.0
20.1
16.6
HR (95% CI)
0.73 (0.57‒0.93)
NA
†
0.96 (0.74‒1.23)
Right
11.1
15.4
17.5
21.0
10.3
8.1
HR (95% CI)
0.87 (0.55‒1.37)
NA
†
1.14 (0.68‒1.89)
Median PFS, months
Left
12.9
9.2
14.6
11.5
8.0
5.8
HR (95% CI)
0.72 (0.57‒0.90)
NA
†
0.88 (0.69‒1.12)
Right
7.5
7.0
8.7
12.6
4.8
2.4
HR (95% CI)
0.80 (0.50‒1.26)
NA
†
0.75 (0.45‒1.27)
CR + PR, %
Left
68
53
64
57
50
13
Right
42
35
64
50
13
3


