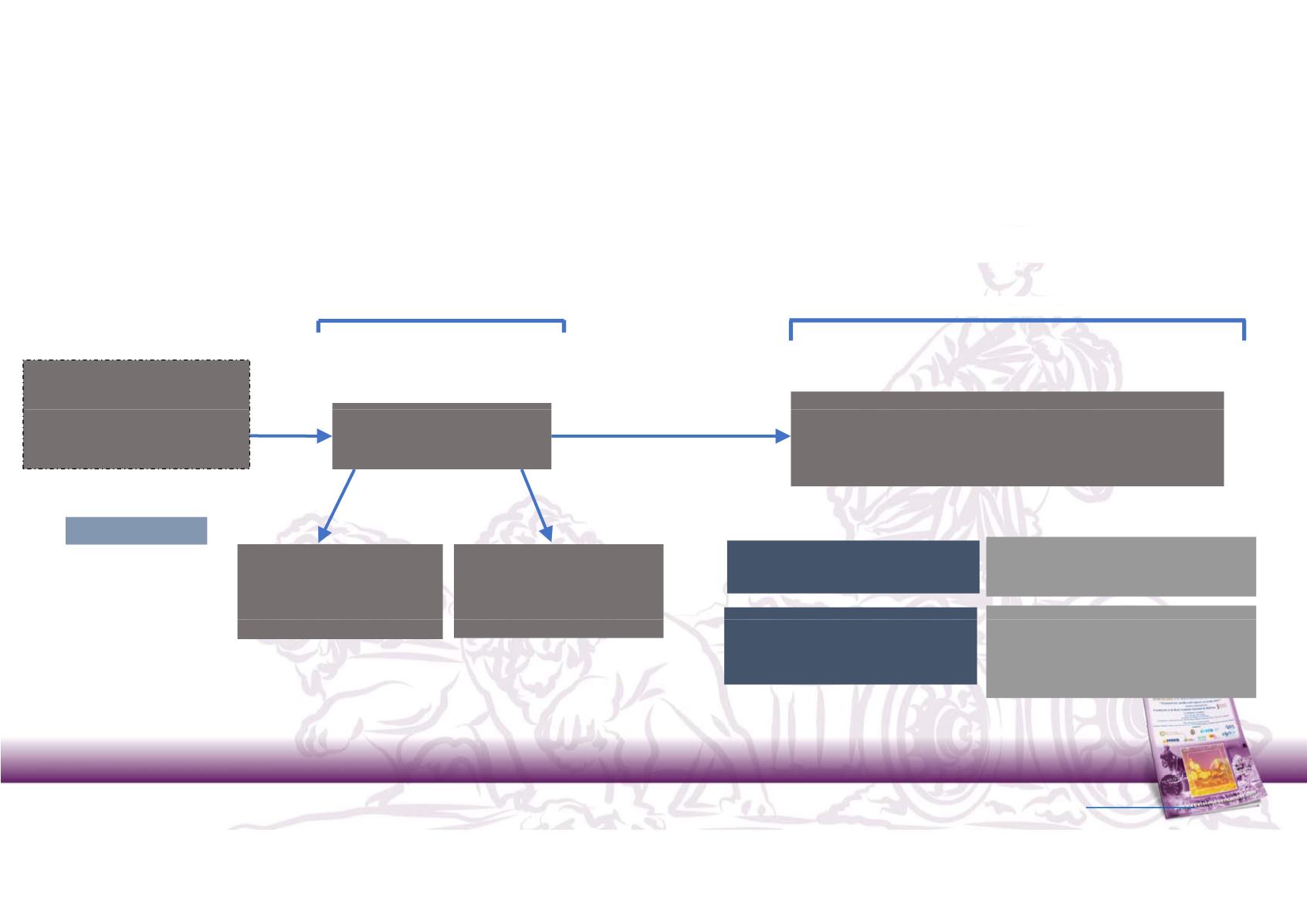
JAVELIN Renal 100: Phase 1b dose‐finding study of avelumab + axitinib in
1
st
line mRCC
Phase Ib Study
Dose finding part
Dose expansion part
7‐day lead‐in period with single agent
axitinib prior to cycle 1
-
-
Treatment until confirmed disease progression, unacceptable toxicity, or
withdrawal
Patients with previously
d RCC l
d
DL1: avelumab 10mg/kg
Q2W + axitinib 5mg BID
DL1: avelumab 10mg/kg Q2W + axitinib 5mg BID
untreate a , unse ecte
for PD‐L1 expression
DL‐1A: avelumab 5mg/kg
Q2W + axitinib 5mg BID
DL‐1B: avelumab
10 mg/kg Q2W + axitinib 3
mg BID
Primary endpoint:
DLTs within the first 4 weeks (2 cycles
of treatment
N = 55
Identification of expansion test dose(s) defined as MTD or RP2D, by
mTPI method
Secondary endpoints:
Safety, OR, PFS, OS, TTR,DC, DR, PK,
Immunogenicity and tumour
biomarkers
NCT02493751


