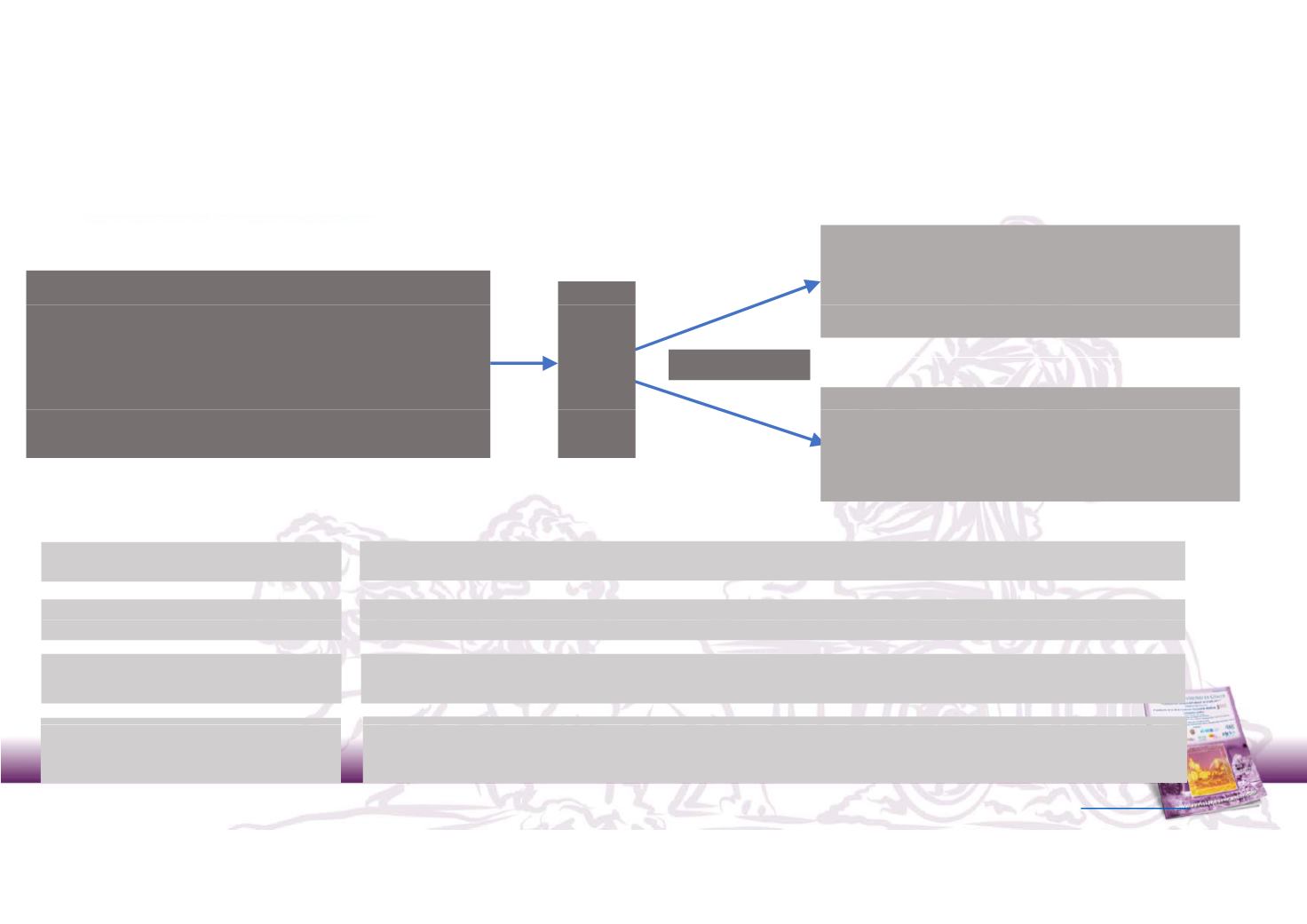
KEYNOTE‐426: Phase 3 study of pembrolizumab + axitinib in 1st line
mRCC
Enrollment Criteria
Arm A
pembrolizumab 200mg Q3W+ axitinib 5mg BID
n
•
Advanced clear cell RCC
•
No prior systemic therapy
•
Measurable disease per RECIST 1.1
•
Adequate organ function
N = 840
ndomisatio
1:1
•
KPS ≥ 70%
Arm B
sunitinib 50mg OD 4/2
Ra
Primary endpoints:
PFS by BIRC and OS
Secondary endpoints
OR DC AEs biomarkers PROs
,
,
,
,
Stratification
IMDC and region
NCT02853331
Trial locations
Brazil, Canada, Czech Republic, France, Germany, Hungary, Ireland, Italy, Japan, Poland, Russia, South
Korea, Spain, Taiwan, Ukraine, United Kingdom, United States


