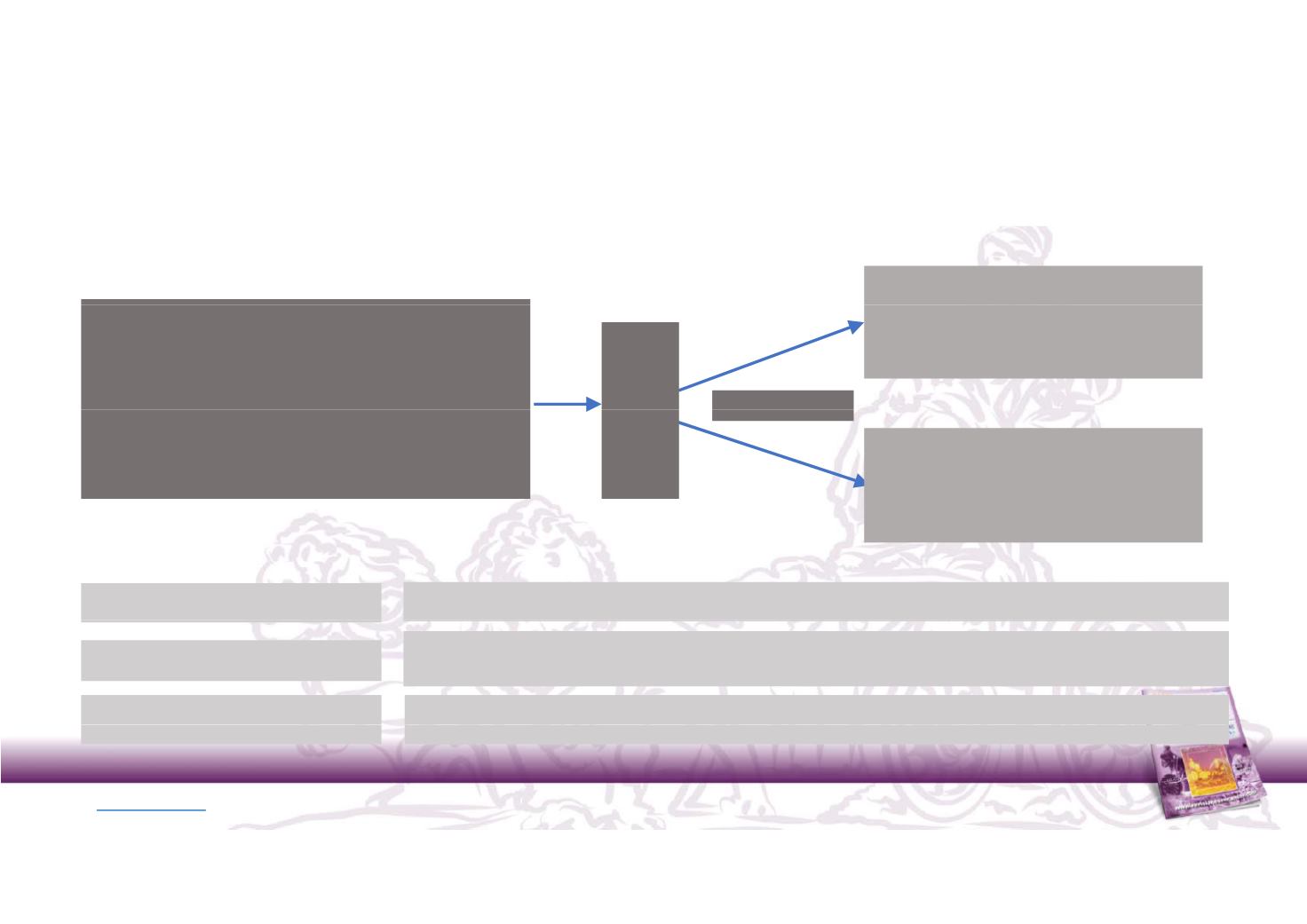
JAVELIN Renal 101:
Phase 3 study of avelumab + axitinib in 1st line mRCC
Arm A
Enrollment Criteria
•
Advanced clear cell RCC
•
No prior systemic therapy
•
At least one measureable lesion by RECIST 1.1
avelumab 10mg/kg Q2W + axitinib 5mg
BID
N = 583
misation
:1
•
ECOG PS 0 or 1
•
Adequate bone marrow function, renal and liver
functions
Arm B
sunitinib 50 mg OD 4/2
Rando
1
Primary endpoint:
PFS by BIRC
Secondary endpoints
OS, OR, DC, TTR, DR, PFS by Investigator
AEs, laboratory abnormalities, PK, ADA, biomarkers, PROs
Stratification
ECOG PS (0 vs. 1) and region (US vs Canada/Western Europe vs ROW)
NCT02684006


