First line trials: closed for recruitment
Hammers HJ, et al. ESMO 2016
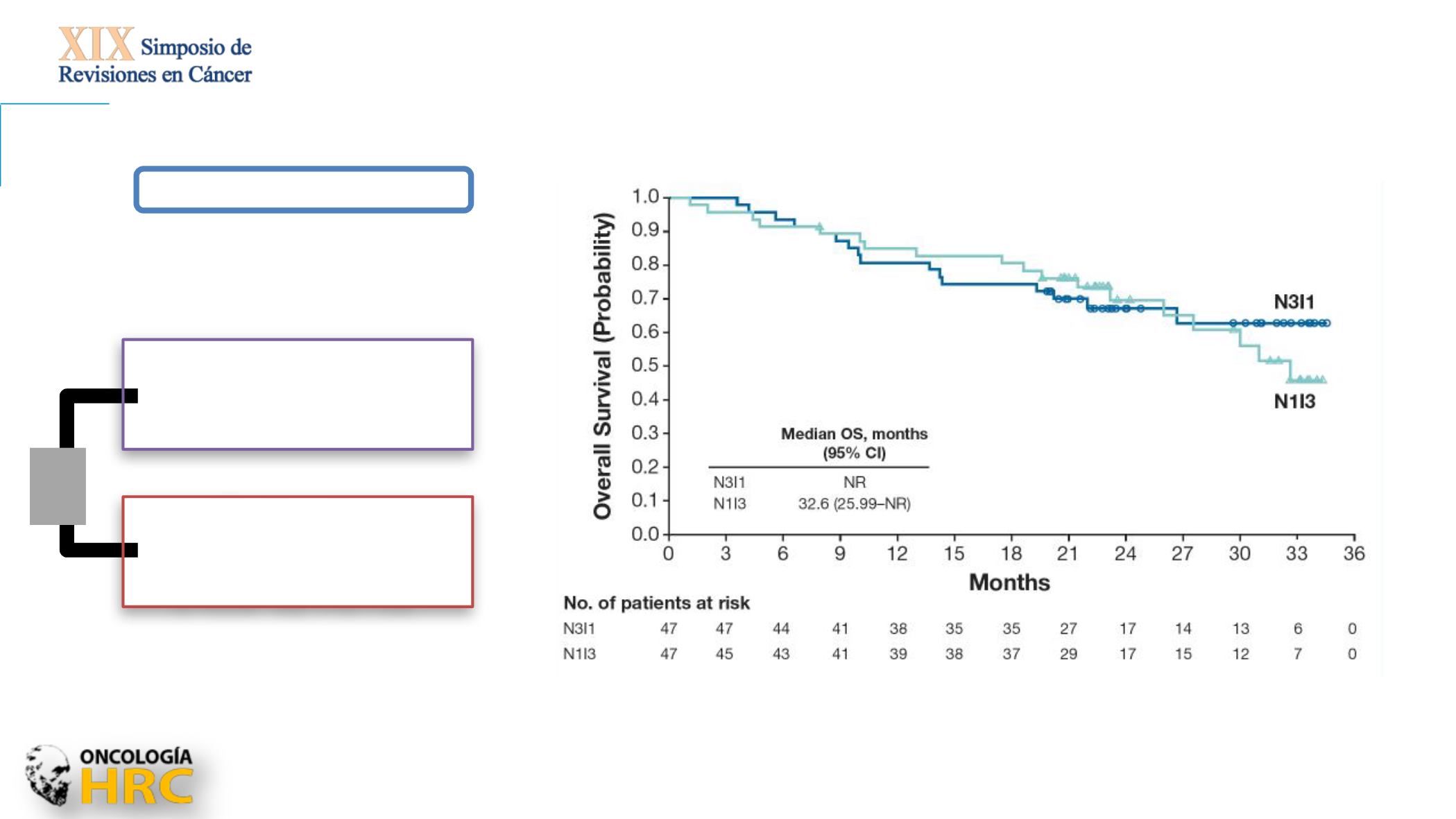
Checkmate214
Phase III
Co-Primary endpoint: PFS,
OS
R
Nivolumab +
ipilimumab
3mg/kg IV + 1mg/kg IV
every 3 weeks X4
then Nivolumab 3mg/kg IV every 2 weeks
Sunitinib
50 mg/day 4/2
PD-1 + CTLA-4 inhibition
n=1071


