PRESENTED AT ESMO 2016.
SLIDES ARE THE PROPERTY OF THE AUTHOR. PERMISSION REQUIRED FOR REUSE.
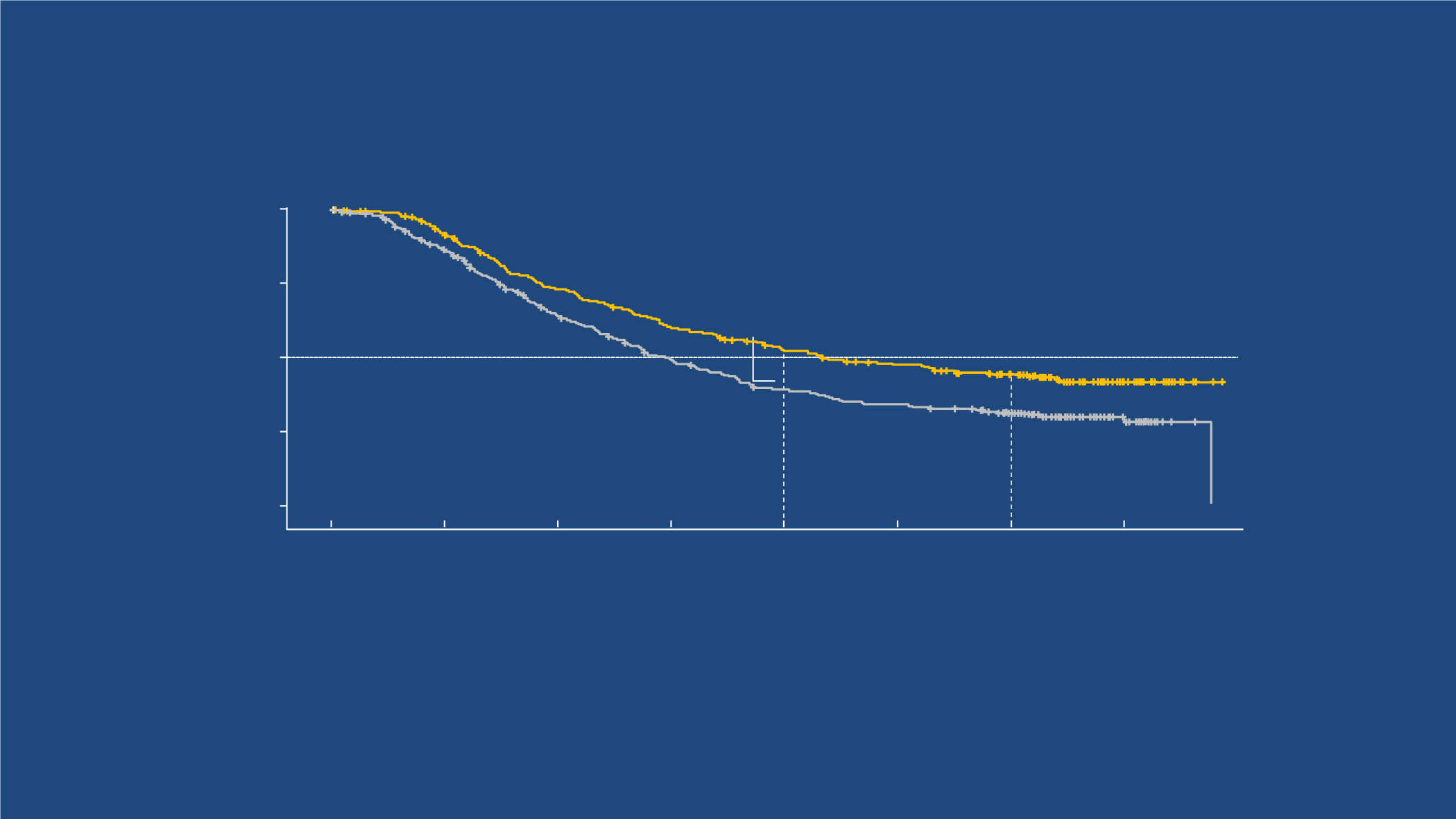
COMBI-v: Overall Survival (ITT population)
12
1.00
0.75
0.50
0.25
0.00
0
6
12
18
24
30
36
42
Months From Randomization
OS Probability
Patients at risk, n
D + T
Vem
352
352
311
289
245
203
201
154
171
119
150
103
127
81
33
22
3-y OS, 45%
3-y OS, 31%
2-y OS, 53%
2-y OS, 39%
Vemurafenib (n = 352)
a
Median OS, 17.8 mo (95% CI, 15.6-20.7)
•
128 censored pts, 89 (70%) ongoing f/u,
of which 10 (11%) are still on study tx
HR, 0.68 (95% CI, 0.56-0.83)
Dabrafenib + trametinib (n = 352)
Median OS, 26.1 mo (95% CI, 22.6-35.1)
•
162 censored pts: 134 (83%) ongoing f/u, of
which 66 (49%) are still on study tx
a
Vemurafenib arm includes 34 patients (10%) who crossed over to the dabrafenib + trametinib arm.
D + T, dabrafenib + trametinib; f/u, follow-up; ITT, intent-to-treat; pts, patients; tx, treatment; Vem, vemurafenib.


