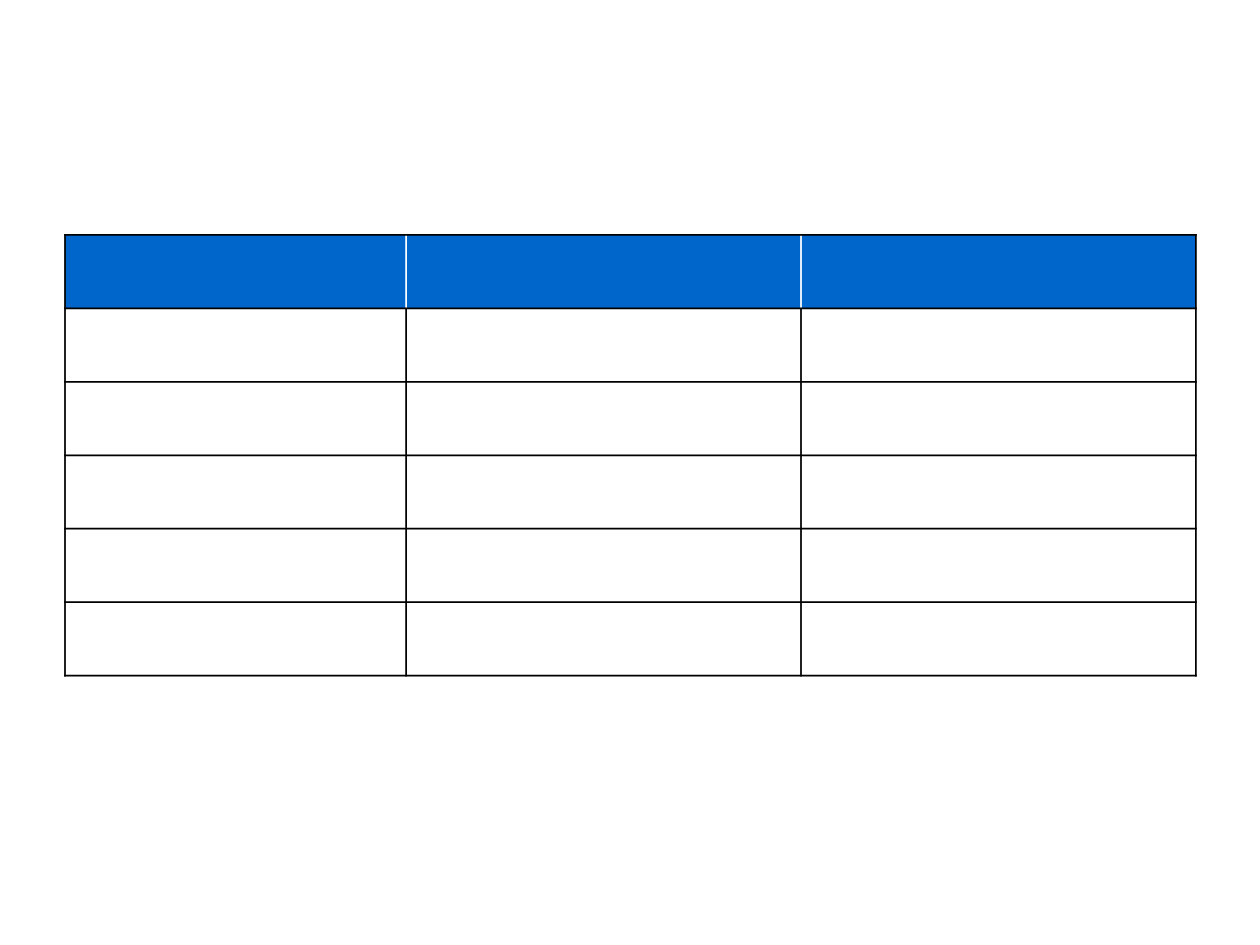
Overview of ceritinib in the crizotinib-failure setting
(phase I and II studies)
SAE = serious adverse event
*By investigator
1. Kim, et al. Lancet Oncol 2016; 2. Crino, et al. J Clin Oncol 2016
ASCEND-1
1
ASCEND-2
2
Number of patients
163
140
Phase
I
II
Objective response rate, %
56*
39*
Median PFS, months
6.9
5.7
Median DoR, months
8.3
9.7
ASCEND-2 safety summary
2
•
Most common AEs: nausea (81%), diarrhoea (80%) and vomiting (60%)
•
8% of patients discontinued due to AEs
•
SAEs regardless of study drug relationship: 41% of patients


