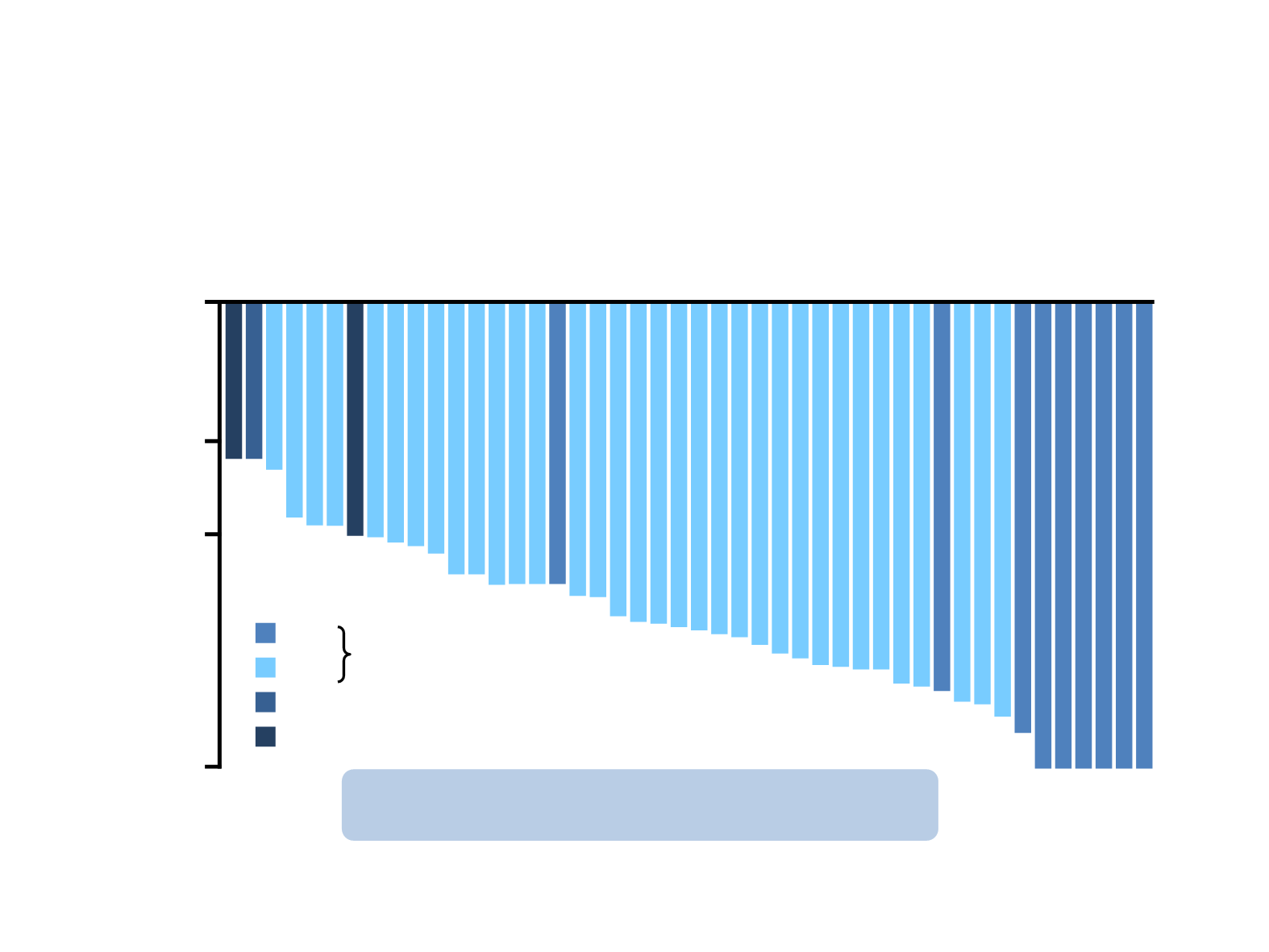
AF-001JP: alectinib in ALK inhibitor-naïve patients
with
ALK
+ disease
*Lymph nodes are identified as target lesion for RECIST evaluation
CR = complete response; NE = not evaluated; PR = partial response; SD = stable disease
31 Jan 2014 cut-off
Please note that alectinib has not yet received regulatory approval in the EU
Tamura, et al. CMSTO 2014; Ohe, et al. ASCO 2015
0
–30
–50
–100
CR
PR
SD
NE
*
*
*
Change from baseline (%)
n=46
ORR = 93.5%
Percentage change in tumour size from baseline (ITT population, by IRC)
Median PFS estimate >29 months
INDICACIÓN NO APROBADA, EN INVESTIGACIÓN


