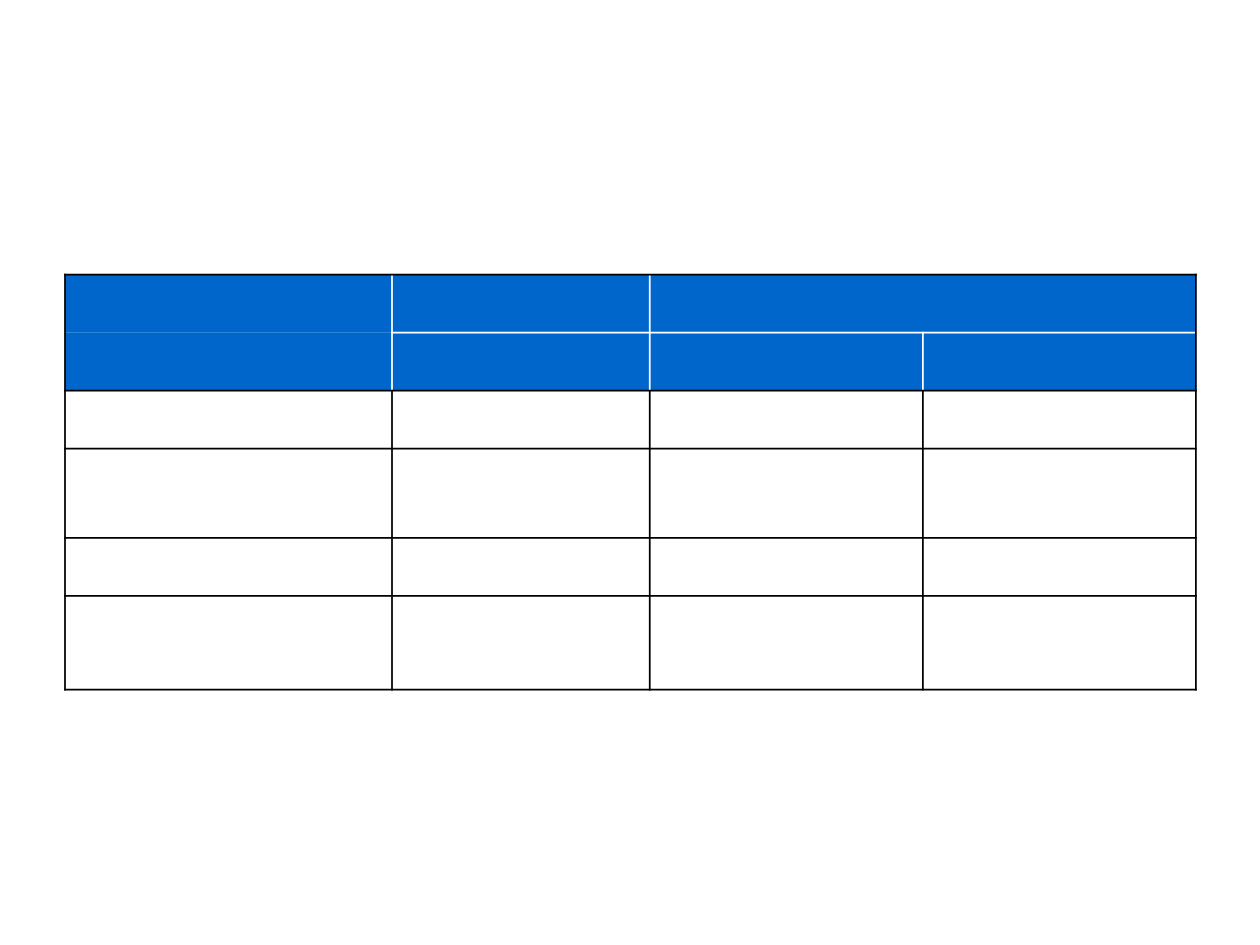
Overview of brigatinib in crizotinib-resistant patients
(phase I and II studies)
1. Bazhenova, et al. ESMO 2016; 2. Kim, et al. ASCO 2016
Phase I/II
1
Phase II (ALTA)
2
90/180mg
90mg
180mg
Number of patients
71
112
110
Objective response rate,
%
62
45
54
Median PFS, months
12.9
9.2
12.9
Intracranial response
rate, %
53
(n=15)
36
(n=25)
67
(n=18)
ALTA safety summary
2
•
Most common AEs with 180mg QD brigatinib: nausea (40%), headache (38%) and cough (34%)
•
Early onset pulmonary events occurred in 6% of patients, all events occurred at 90mg QD in both arms, no
events were observed after escalation to 180mg
̶
the pathophysiology of these events is unclear
INDICACIÓN PENDIENTE DE APROBACIÓN,


