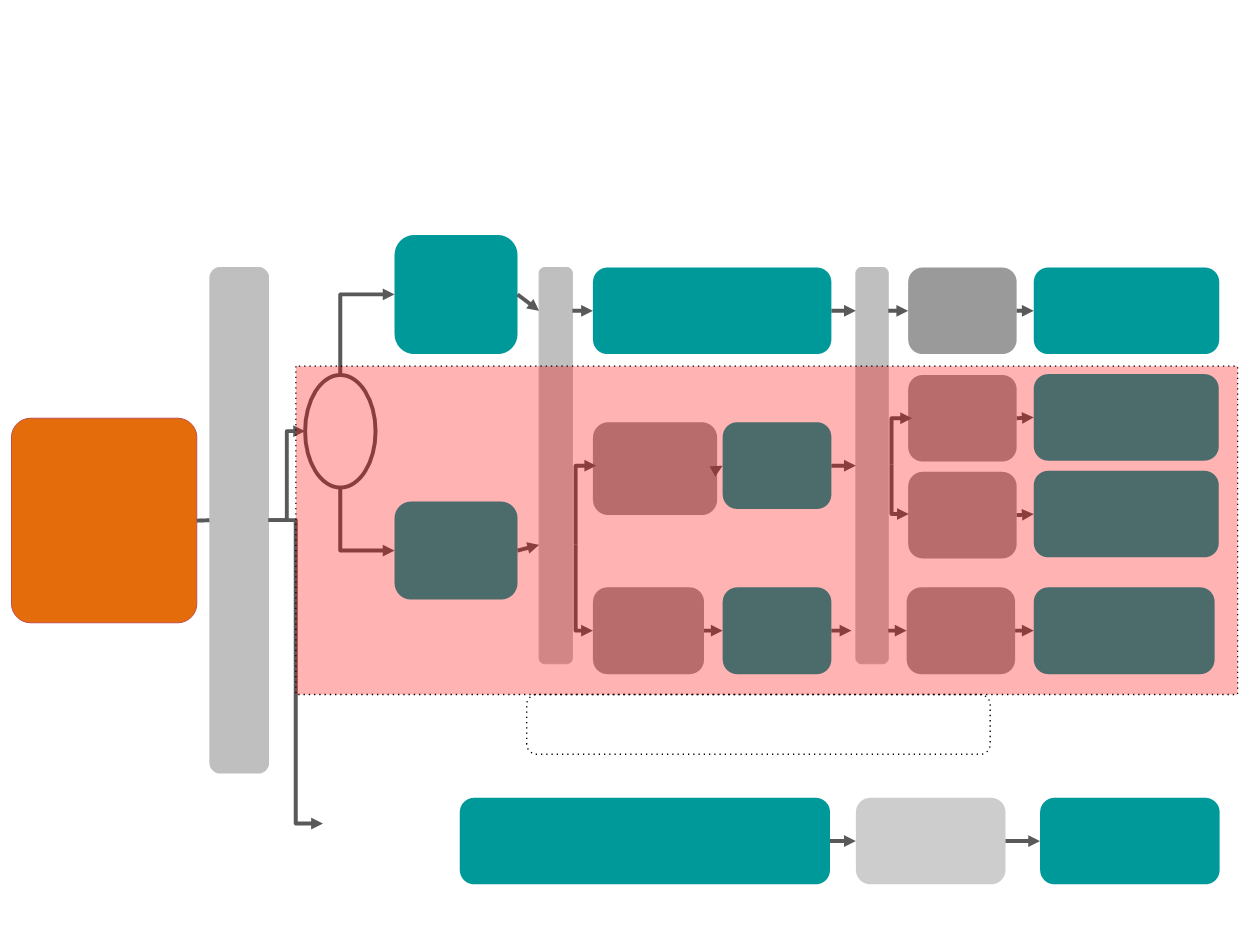
PHERGain – MEDOPP096
A randomised, non-comparative phase II
study to de-escalate chemotherapy
•
Operable, locally
advanced or
inflammatory
•
HER2+ EBC
•
Tumour size
>2cm
•
18 years
•
ECOG 0/1
•
No prior
treatment
•
Central analysis
(HER2)
PET
Responders
(sensitive)
PH (ETx)
x2
§
PET-sensitive: RECIST responder with SUV
reduction
>=
25%
PET
Non-
responders
(resistant)
PH (ETx)
x6
PH+CD
x6
SURGERY
pCR
PH (ETx)
x10
pCR or
non-pCR
Non-pCR
PH+CD x6 –
PH (ETx) x4
PH (ETx)
x10
PH+CD
x2
PH+CD
x4
PH (ETx)
x12
BASAL: PET/CT Scan (Total Body) Breast MRI / Biopsy
PET/CT Thoracic Scan
WEEK
6
N=355
N=284
N=71
pCR or
non-pCR
PH+CD
x6
PH (ETx)
x12
If Subclinic
PET M1
N ≤45
Surgery or no
surgery
R*
1:4
COHORT A:
COHORT B:


