Gradishar et al JCO 2005
Nab-paclitaxel: pivotal trial
•
International phase III RCT: Nab-paclitaxel
260
vs paclitaxel
175
q21d
•
Total N=460
•
1L (42 vs 40% per arm)
•
83% postM; 88% previous Rx; 77% previous AC (50% in MBC)
•
Primary endpoint: RR non-inferiority (secondary: TTP, OS, safety)
•
RR: Overall 33 vs 19%* and
1L MBC 42 vs 27%*, p=0.029
•
TTP:
Overall 23 vs 16.9 wk HR 0.75* and
1L MBC 24 vs 19.7 wk (NS)
•
OS:
Overall 65 vs 55.7 wk (NS), >2L MBC 56.4 vs 46.7 wk HR 0.73* and
1L 18 vs 16 mo (NS)
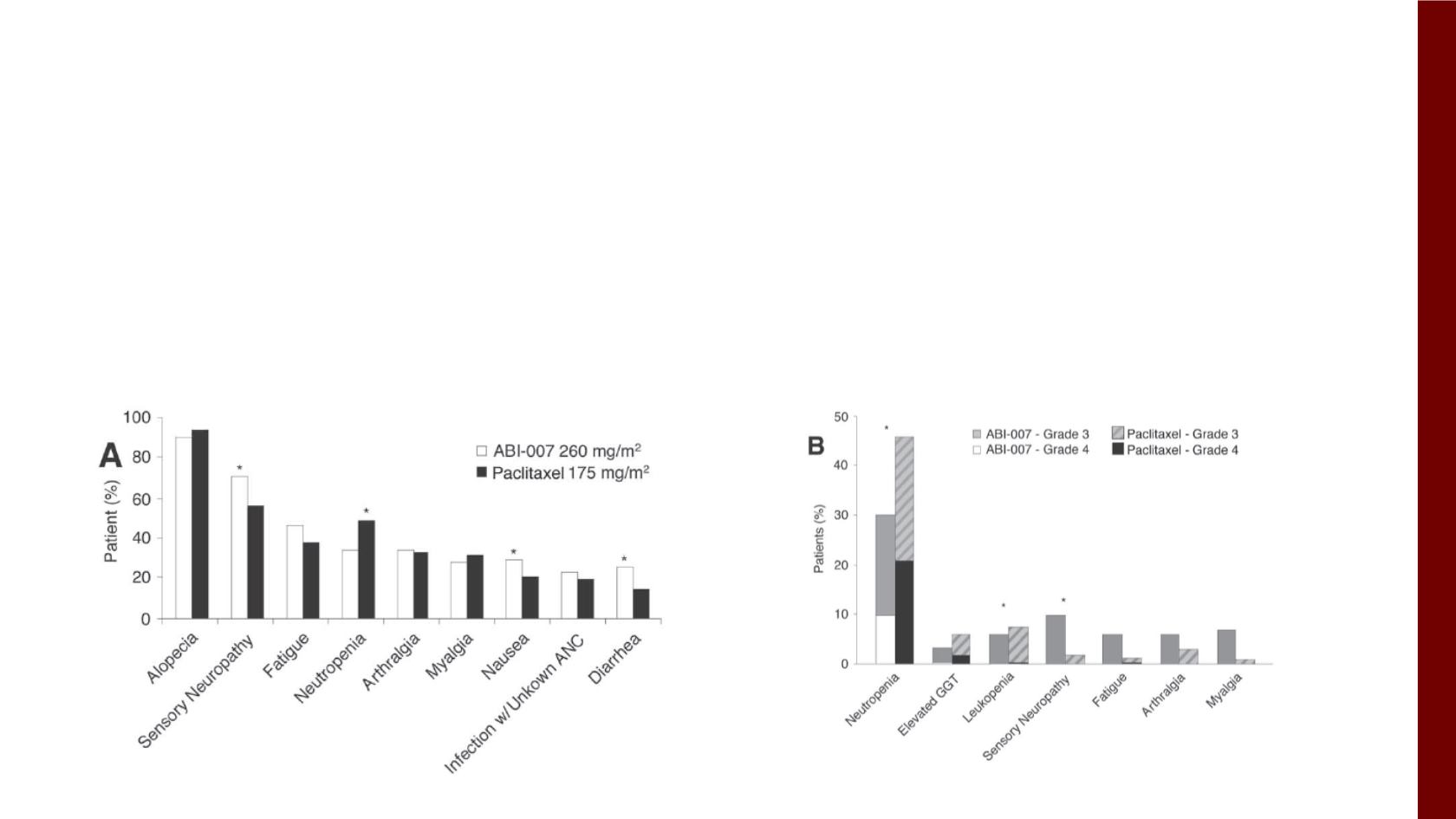
Treatment related AEs (G3/4) >5% of patients
AEs (all grades) > 20% of patients


