17
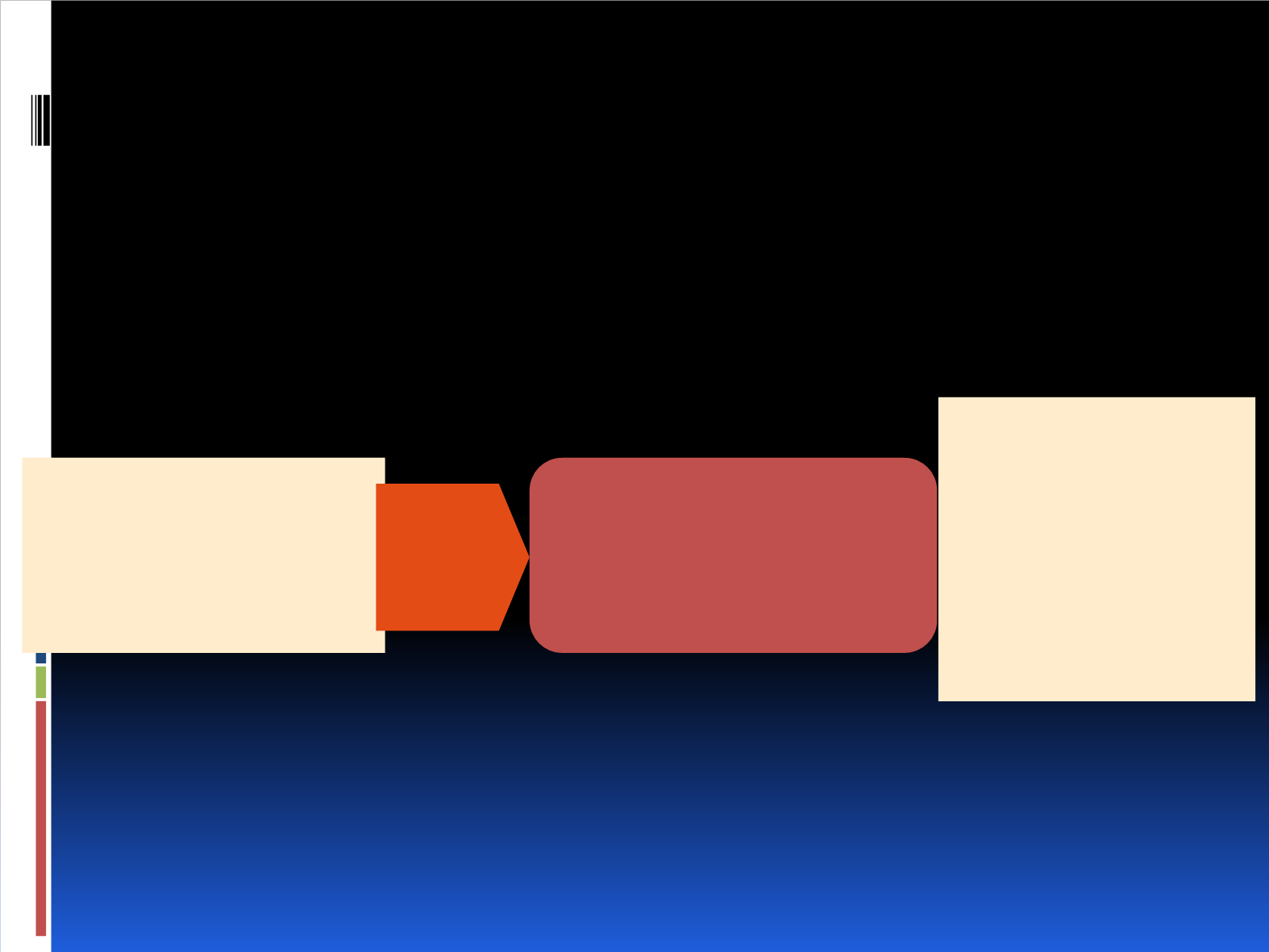
US-based, multicenter, single-arm, phase 2 prevention trial (NCT02069093)
23 investigational sites
Enrollment: May 2014 to October 2015
a
Roxane Laboratories/West-Ward Pharmaceuticals, Eatontown, NJ.
b
At the completion of cycle 2 (day 56), the treating clinician determined whether to continue the patient’s assigned mouthwash regimen. Patients could continue to receive their mouthwash regimen from Novartis
for an additional 56 days.
ECOG: Eastern Cooperative Oncology Group; EVE: everolimus; EXE: exemestane; HER2: human epidermal growth factor receptor 2-negative; HR: hormone receptor; NDS,
Normalcy of Diet Scale; QID, four times a day; VAS, visual analog scale
Treatment cycles (cycle 1, 2), optional cycles (cycles 3, 4)
b
, and
safety follow-up cycle; each cycle = 28 days
N = 92
• Females ≥18 years
• Postmenopausal locally advanced or
metastatic HR+, HER2- breast cancer
• Prescribed EVE 10 mg + EXE 25 mg
• ECOG performance status ≤ 2
Endpoints
Primary:
compare grade ≥2
stomatitis incidence at 8 weeks
with BOLERO-2 results
Secondary:
Mouthwash use by
average times/day, EVE/EXE
dose intensity, all-grade
stomatitis incidence and time to
resolution to grade ≤1, oral pain
scale, normalcy of diet
Baseline
•
Oral pain
assessment
• VAS score
• NDS score
• Routine good oral care
• EVE 10 mg/day
• EXE 25 mg/day
• Alcohol-free steroid-based mouthwash
a
−
10 mL (0.5 mg/5 mL dexamethasone
oral solution) swish for 2 mins and
spit QID
Open label, phase II, single arm


