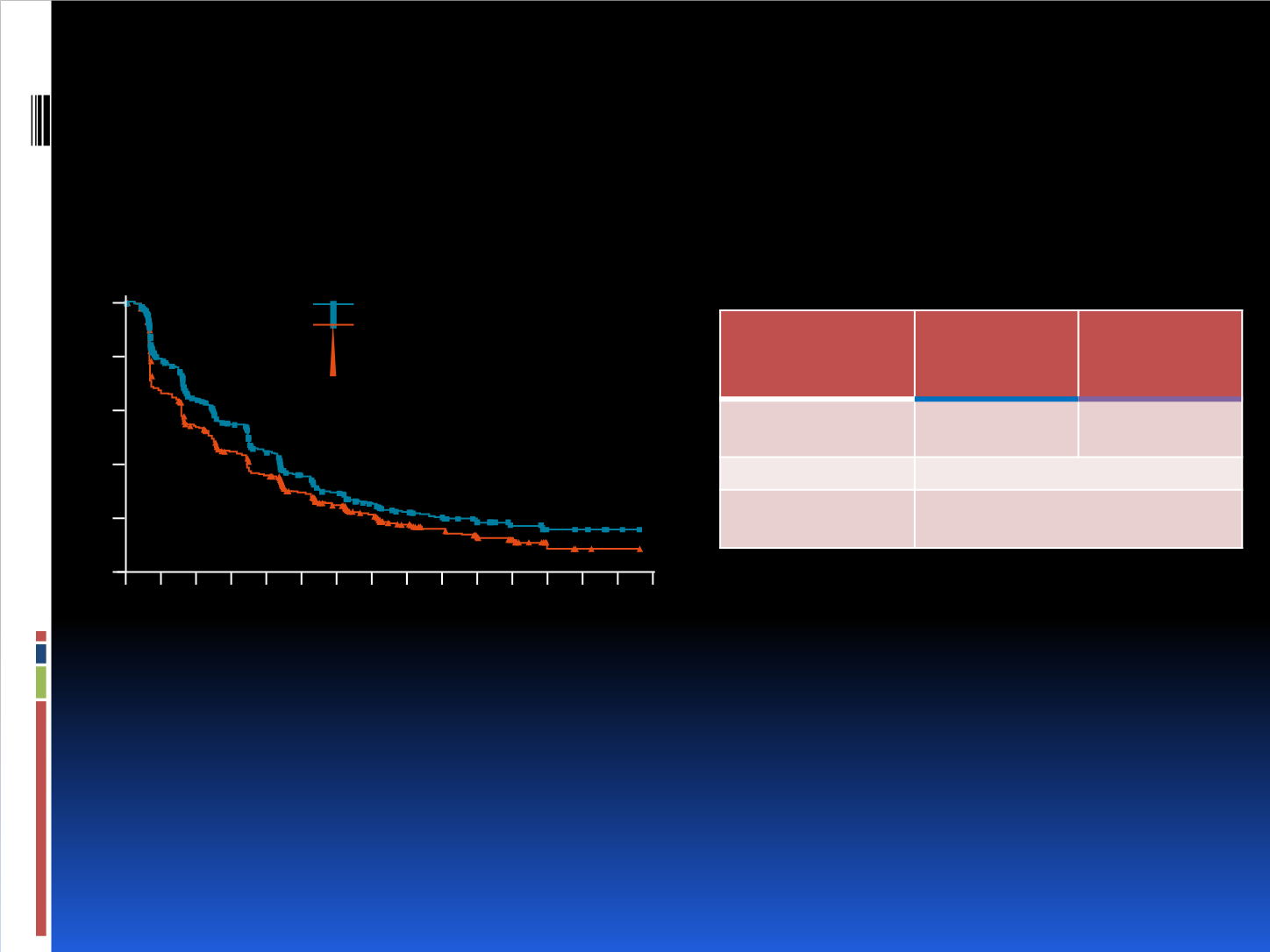
BELLE-2 Met the Primary
Endpoint for PFS Improvement
in the Full Population
A similar PFS improvement was observed in the main population (HR 0.80 [95%CI: 0.68–0.94]; one-sided P
value 0.003)
Follow-up for OS analysis is ongoing, with a pre-specified target of 588 deaths in the full population
–
At the time of primary PFS analysis, OS data were immature (281 deaths in the full population), with a trend in favor of the
buparlisib arm
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Probability of
Progression-free Survival, %
Time (Months)
100
60
0
80
40
20
0
4
8
14
18
2
6
10 12
16
20
26
30
22 24
28
Buparlisib + fulvestrant (n/N=349/576)
Placebo + fulvestrant (n/N=435/571)
Full Population
(N=1147)
Buparlisib +
Fulvestrant
n=576
Placebo +
Fulvestrant
n=571
Median PFS,
months (95%CI)
6.9
(6.8–7.8)
5.0
(4.0–5.2)
HR (95%CI)
0.78 (0.67–0.89)
One-sided
P value
<0.001
1
6
0
9
0
4
3
6
8
1


