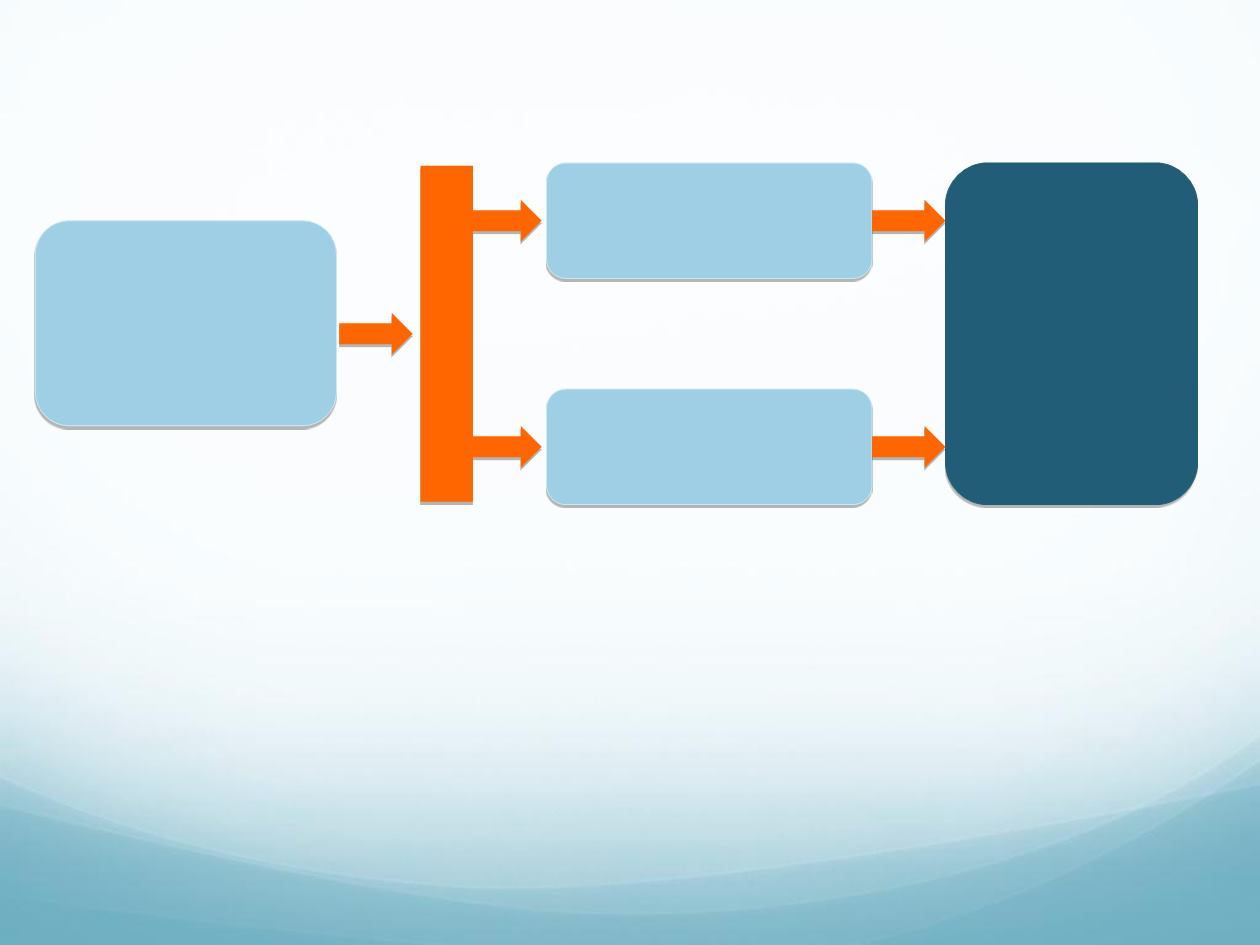
CORRECT study design
Multicenter, randomized, double-blind, placebo-controlled, phase III
2:1 randomization
Strat. factors: prior anti-VEGF therapy, time from diagnosis of mCRC, geographical region
Global trial: 16 countries, 114 active centers
1,052 patients screened,
760 patients randomized within 10 months
Secondary endpoints: PFS, ORR, DCR
Tertiary endpoints: duration of response / stable disease, QOL, pharmacokinetics, biomarkers
No crossover between treatment arms was allowed
Progressive
mCRC during or
within 3 m of the
last standard
therapy
R
A
N
D
O
M
I
Z
A
T
I
O
N
Regorafenib + BSC
160 mg orally once daily
3 weeks on, 1 week off
Placebo + BSC
3 weeks on, 1 week off
2 : 1
Primary
Endpoint: OS
90% power to
detect 33.3%
increase
(HR=0.75), with
1-sided overall
a
=0.025


