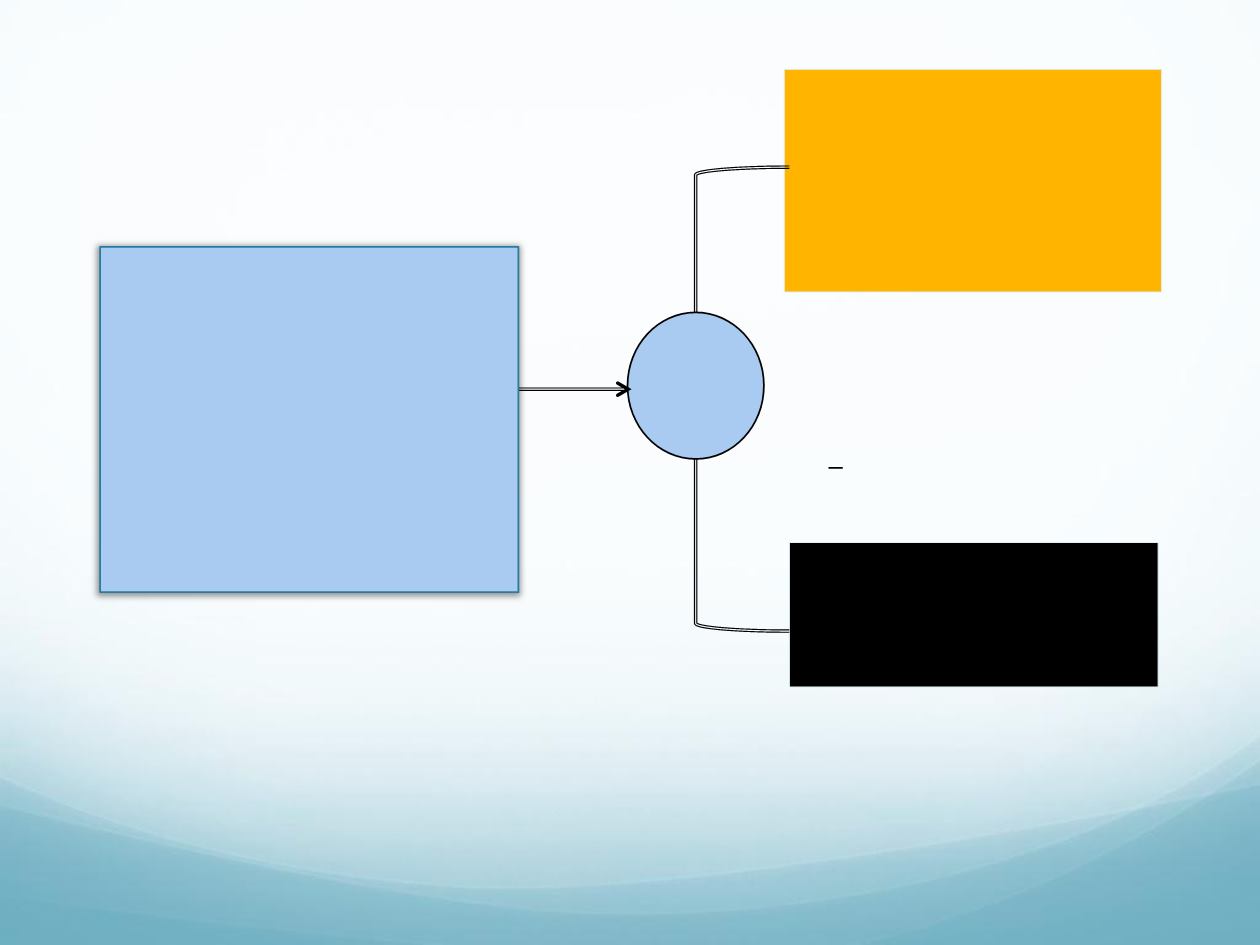
Regorafenib
160 mg daily
3 weeks on / 1 week off
(4-week cycle)
n
= 136
Placebo
n
= 68
Stratification
• Metastases: single
vs
multiple
• Time from mCRC diagnosis:
>18
vs
<18 months
• All patients received best
supportive care
• Treat until progression,
unacceptable toxicity, or
withdrawal
Asian patients with mCRC
who progressed after
standard therapies
25 Centers: mainland China,
Hong Kong, South Korea,
Taiwan, Vietnam
CONCUR trial design
Clinicaltrials.gov NCT01584830
Primary endpoint: overall survival
(OS)
•
One-sided alpha 0.2 and assumed 33.3% OS improvement
(HR=0.75 favoring regorafenib) with 154 events had 80%
power
Secondary endpoints: progression-free
survival, response rate , disease control
rate
R
2:1


