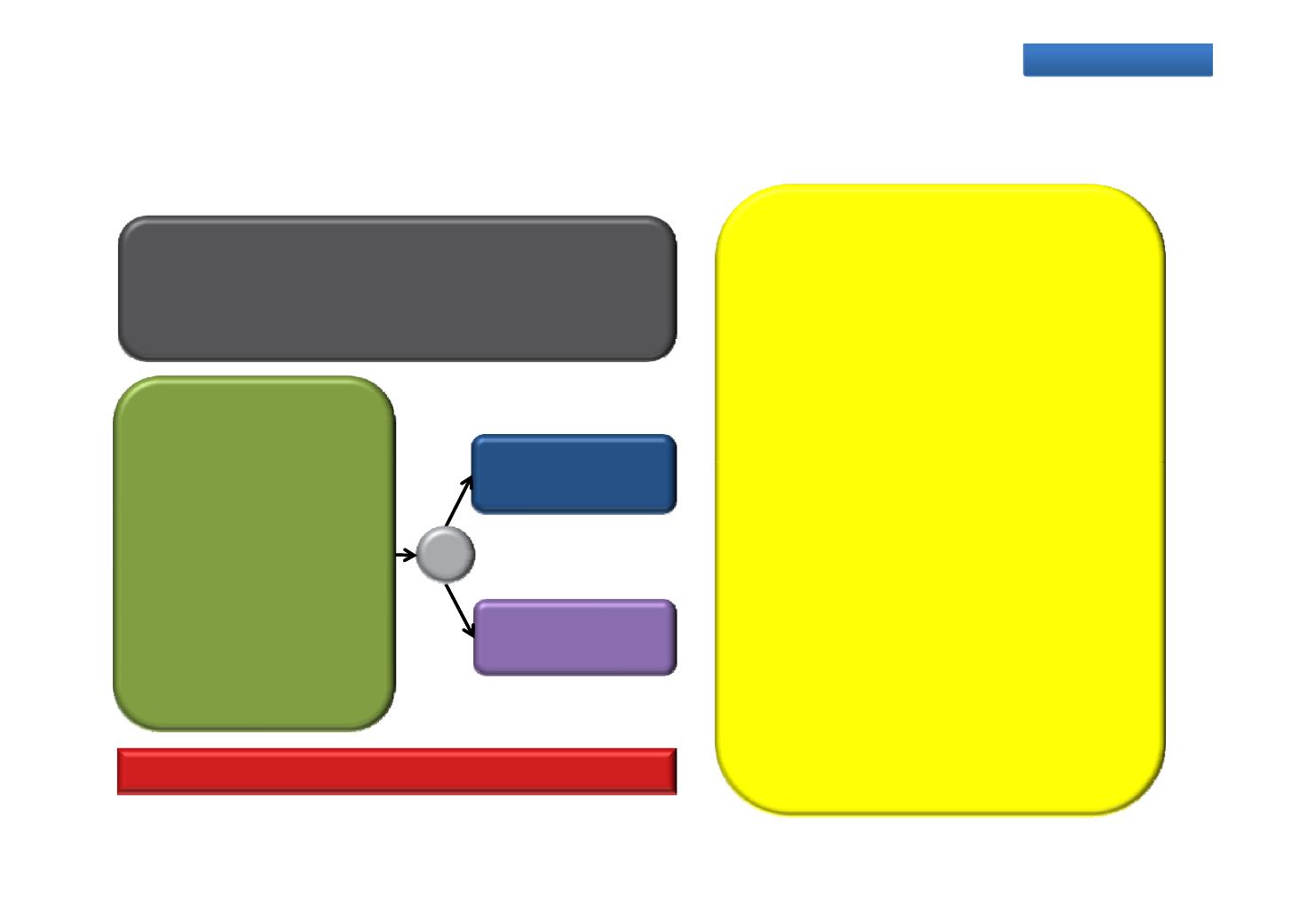
PROSPER: An efficacy and safety study of enzalutamide in
patients with non-metastatic CRPC
Ongoing trials
•
PROSPER is a multinational,
Planned evaluations
Phase 3, randomised, double‐blind,
placebo‐controlled trial
•
Primary endpoint: Metastasis‐free survival
•
Metastasis‐free survival
•
OS
•
Time to pain progression
Enzalutamide
n=1560
Non‐metastatic (M0)
•
Time to opiate use for prostate
cancer pain
•
Time to first use of cytotoxic
h th
160 mg QD
R
2:1
CRPC
Testosterone
≤50 ng/dL
c emo erapy
•
Time to first use of new
antineoplastic therapy
•
Time to PSA progression
Placebo
QD
Progressive disease
with ongoing ADT
Asymptomatic
•
PSA response rates
•
QoL
PSADT ≤10 months
Recruiting
Timing
•
Estimated study completion
August 2017
ADT=androgen‐deprivation therapy; CRPC=castration‐resistant prostate cancer; OS=overall survival; PSA=prostate‐specific antigen;
PSADT=PSA doubling time; QD=once daily; QoL=quality of life; R=randomisation.
NCT02003924. Available at
Last accessed: July 2016.
8


