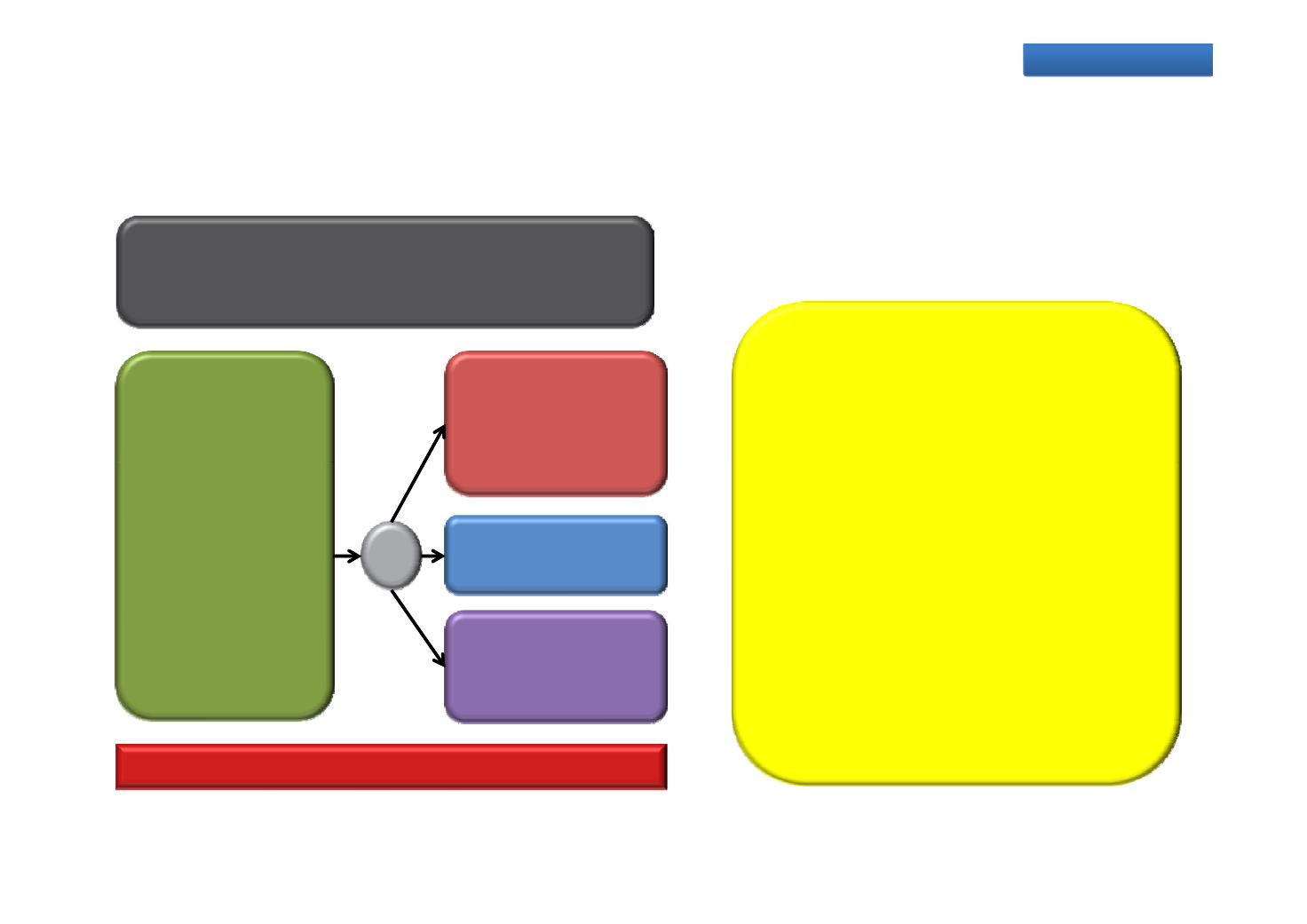
EMBARK: Efficacy and safety of enzalutamide
in patients with high-risk non-metastatic prostate cancer progressing
Clinical trials
after definitive therapy (M0 BCR)
•
Phase 3, randomised, double‐blind, placebo‐
controlled study
•
Primary endpoint: Metastasis‐free survival
Planned evaluations
n=1860
Non‐metastatic
prostate cancer
Enzalutamide
160 mg QD +
leuprolide
12 kl
•
Metastasis‐free survival
•
OS
•
Proportion of patients per group
treatment‐free 2 years after
Hormone‐
sensitive
High‐risk
Enzalutamide
160 QD
‐wee y
R
1:1:1
suspension of study drug
•
Time to castration resistance
•
Prostate cancer‐specific survival
Ti
fi
i SRE
Progression
following
definitive
mg
Placebo +
leuprolide
•
me to rst symptomat c
•
Safety (AEs, vital signs,
laboratory evaluations)
radiotherapy
12‐weekly
Recruiting
Timing:
Estimated study completion
December 2020
AE=adverse event; BCR=biochemical relapse; QD=once daily; OS=overall survival; R=randomisation; SRE=skeletal‐related event.
NCT02319837. Available at
. Last accessed: august 2016
1
4


