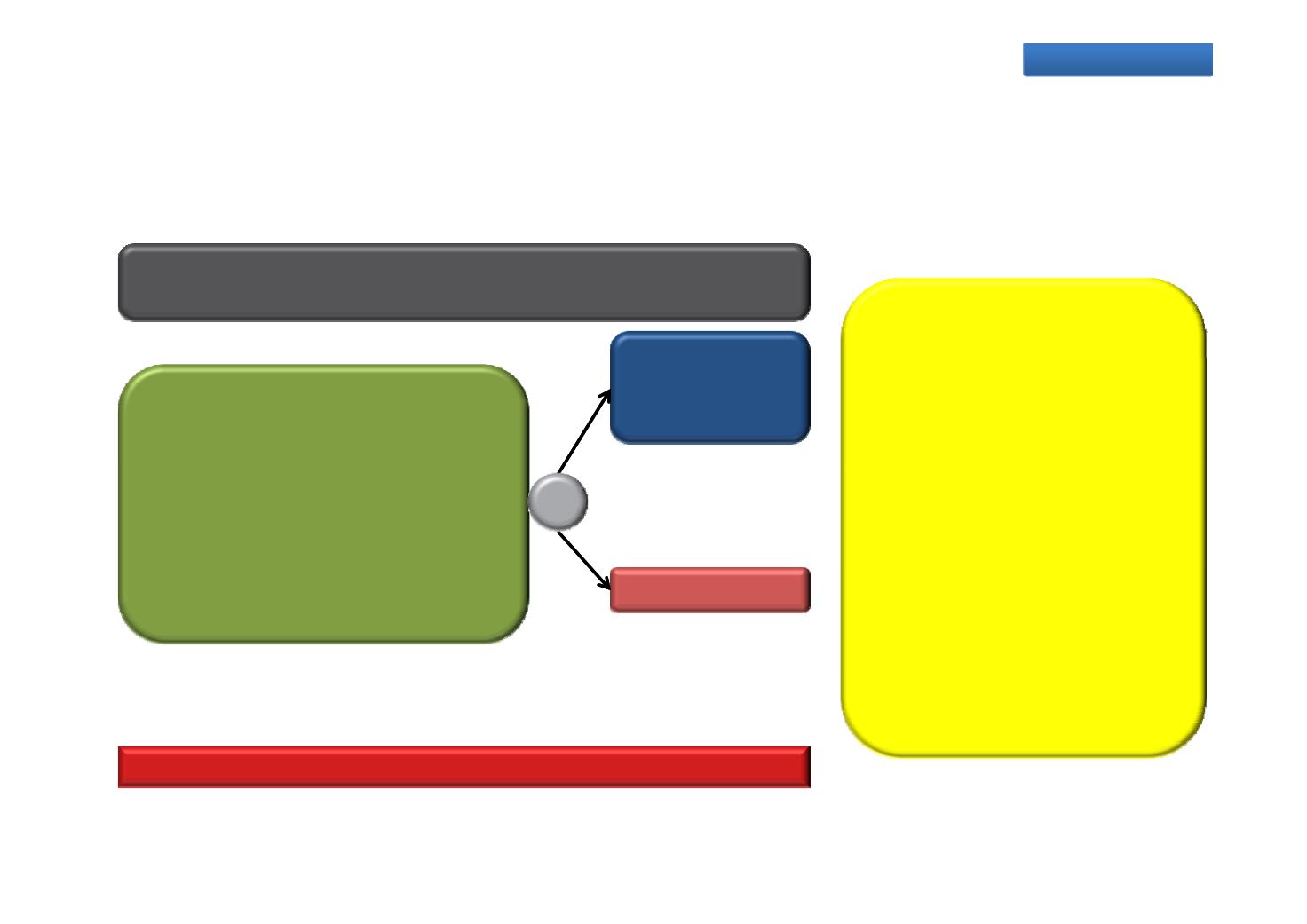
ARCHES: A Multinational, Phase 3, Randomized, Double-blind, Placebo-controlled Efficacy and Safety
St d f E l t id Pl A d D i ti Th
(ADT) V Pl
b Pl ADT i P ti t
Ongoing trials
u y o nza u am e us n rogen epr va on erapy
ersus ace o us
n a en s
with Metastatic Hormone Sensitive Prostate Cancer (mHSPC)
Enzalutamide
•
A Phase 3 multicentre study
•
Primary endpoint: rPFS
Planned evaluations
‐OS
160 mg QD
+ ADT
n=1100
Metastatic HSPC (bone scan or CT)
‐Time to first SSE
‐Time to castration resistance
‐Time to deterioration of QoL
‐Time to initiation of new
R
antineoplastic therapy
‐Time to PSA progression (? 2
ng/mL) (Prostate Cancer Clinical
Trials Working Group 2 criteria)
No prior pharmacotherapy, radiation
therapy or surgery for metastatic
prostate cancer
Up to 6 cycles of docetaxel therapy
ECOG PS 0–1
Placebo + ADT
‐PSA undetectable rate (< 0.2
ng/mL)
‐
ORR
‐Time to pain progressionTiming
allowed
RECRUITING
Estimated study completion
December 2023
15
NCT02677896 Available at
.


