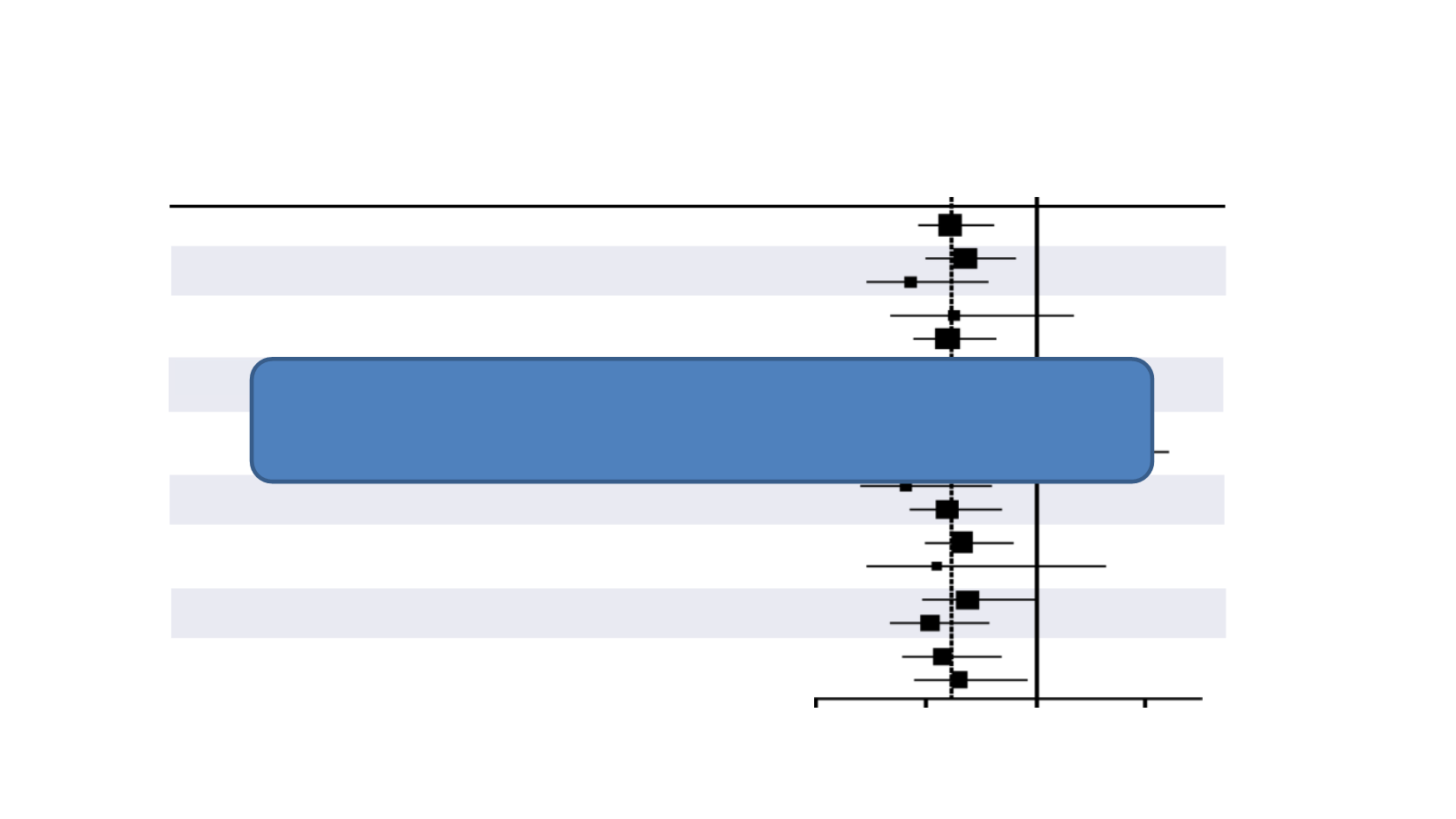
Group
N
HR
95% CI
(0.47-0.80)
(0.50-0.91)
(0.23-0.78)
(0.34-1.17)
(0.45-0.81)
(0.23-0.99)
(0.46-0.91)
(0.47-0.83)
(0.17-1.60)
(0.21-0.80)
(0.43-0.84)
(0.50-0.89)
(0.23-1.31)
(0.49-0.99)
(0.34-0.79)
(0.34-0.79)
(0.45-0.96)
0.61
0.68
0.43
0.63
0.60
0.48
0.65
0.62
0.53
0.41
0.60
0.66
0.55
0.69
0.52
0.58
0.65
790
612
178
276
514
125
387
674
92
220
480
575
214
459
331
443
347
All patients
Age <70
Age ≥70
Low-volume disease
High-volume disease
Visceral mets ± bone mets (BM)
High volume (BM only)
Race - white
Race - other
Gleason score <8
Gleason score ≥8
Prior local therapy - no
Prior local therapy - yes
CAB >30 days - no
CAB >30 days - yes
SRE - no
SRE - yes
CHAARTED – Initial OS analysis
Sweeney CJ et al. New Engl J Med 2015;373:737-46
0
0.5
1.0
1.5
Favor ADT+DOC
BM: bone metastases; CAB: complete androgen blockade; HR: hazard ratio; SRE: skeletal-related event
Age ≥70 years: HR = 0.43 (0.23-0.78)
SAEU.CAB.16.07.0040j


