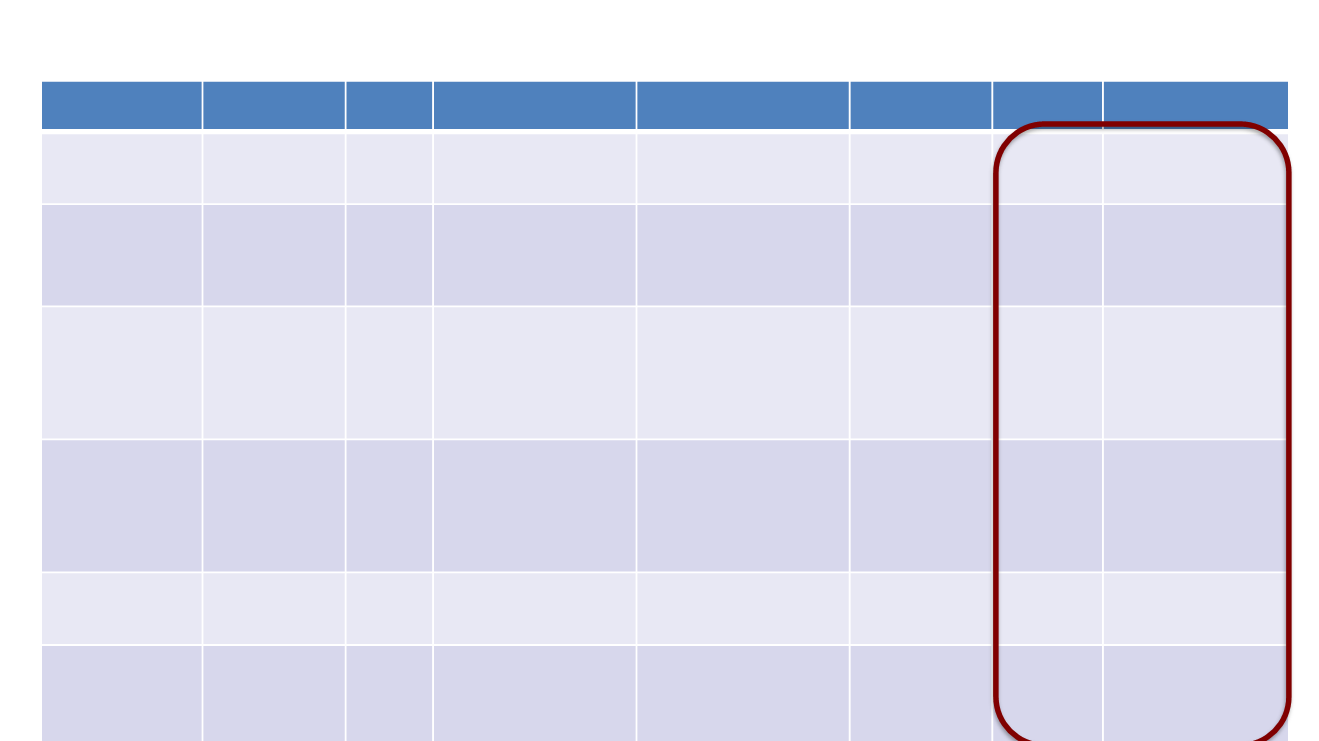
Phase III clinical trials in mCRPC
Study
Agents
N
Indication
HR [95% CI]
∆ OS
Age
Patients > 75 a
TAX-327
1
DOC/P vs
mitox/P
1,006 mCRPC
0.76 [0.62-0.94]
+2.9
68
20%
IMPACT
2
Sipuleucel-
T vs pbo
512
mCRPC (pre-
DOC)
0.78 [0.61-0.98]
+4.1
72
NR
COU-AA-302
3
COU-AA-301
4
ABI/P vs P
ABI/P vs P
1,088
1,195
mCRPC (pre-
DOC)
mCRPC (post-
DOC)
0.81 [0.70-0.93]
0.74 [0.64-0.86]
+4.4
+4.6
71
69
34%
28%
PREVAIL
5
AFFIRM
6
ENZA vs
pbo
ENZA vs
pbo (or P)
1,717
1,199
mCRPC (pre-
DOC)
mCRPC (post-
DOC)
0.77 [0.67-0.88]
0.63 [0.53-0.75]
+4.0
+4.8
72
69
36%
25%
TROPIC
7
CABA/P vs
mito/P
755
mCRPC (post-
DOC)
0.70 [0.59-0.83]
+2.4
67
19%
ALSYMPCA
8
Radium-
223 vs pbo
921
mCRPC (post-
DOC or unfit for
DOC)
0.70 [0.55-0.88]
+2.8
71
28%
SAEU.CAB.16.07.0040j


