6
•
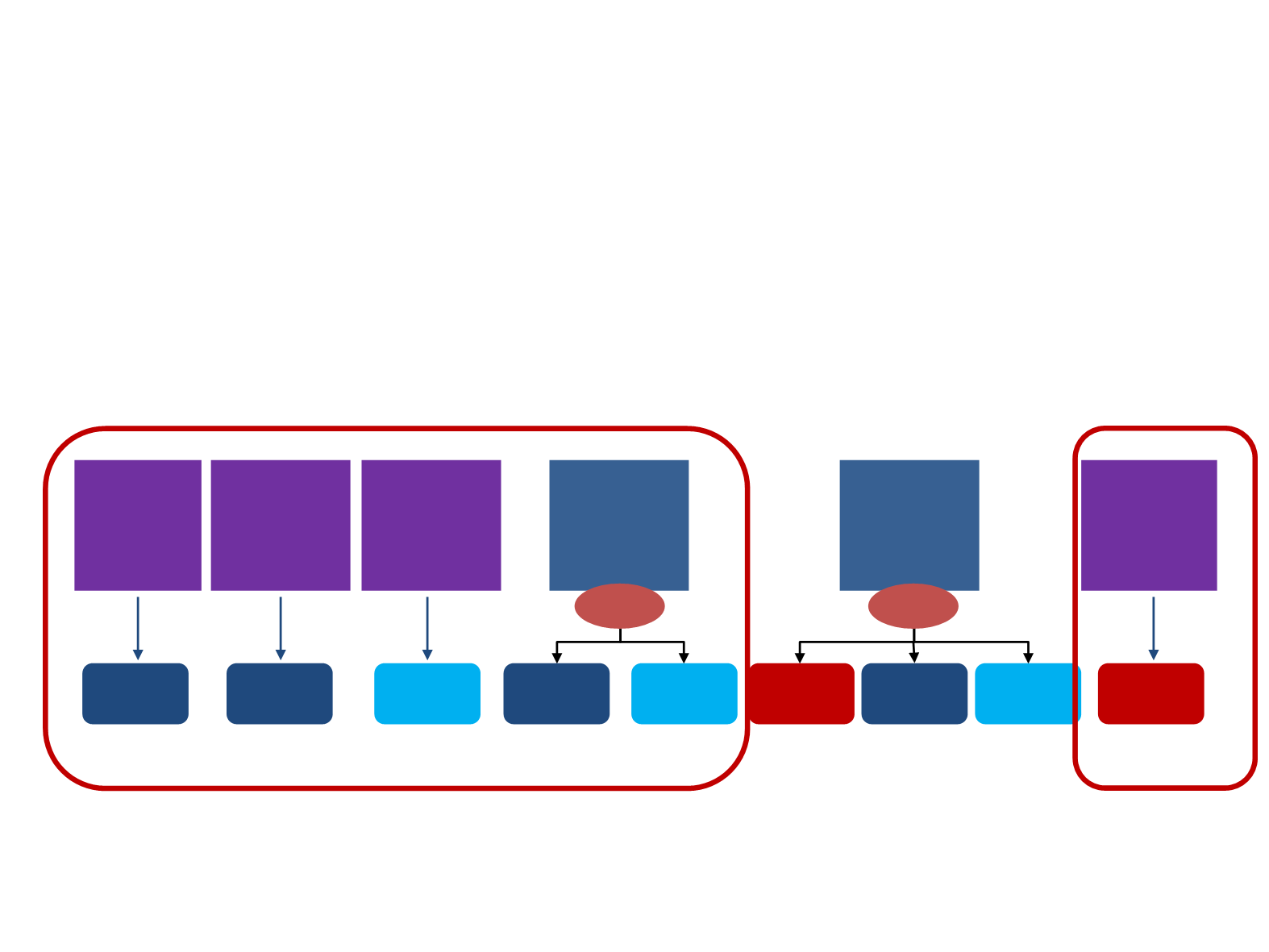
KN-001, Phase I Study initially designed to determine the most effective dose and schedule for pembrolizumab in
patients with multiple tumor types, including NSCLC
1
•
Early data from the trial (N=38) showed enhanced clinical benefit in patients with NSCLC determined to be positive for
PD-L1 expression by a prototype IHC assay
2
•
KN-001 thus evolved into a larger phase 1 trial (N=550) incorporating training and validation sets in order to define
and validate a tumor PD-L1 expression level that would permit a better approach to treating patients
1
Non-
randomized
(N=38)
≥2 prior
therapies
Pembro 10
mg/kg
Q3W
1. Garon EB et al.
N Engl J Med
. 2015;372(21):2018–2028. 2. Gandhi L et al.
Cancer Res.
2014;74:CT105.
Non-randomized
(N=33)
PD-L1+ tumor
≥2 prior
therapies
Non-randomized
(N=43)
PD-L1- tumor
≥1 prior therapy
Randomized
(N=280)
PD-L1+ tumor
≥1 prior therapy
Pembro 10
mg/kg
Q3W
Pembro 10
mg/kg
Q2W
R
(3:2)
Pembro 10
mg/kg
Q3W
Pembro 10
mg/kg
Q2W
Randomized
(N=101)
PD-L1+ tumor
Treatment naive
Pembro
2 mg/kg
Q3W
Pembro 10
mg/kg
Q2W
R
(1:1)
Pembro 10
mg/kg
Q3W
Non-randomized
(N=55)
PD-L1+ tumor
≥1 prior therapy
Pembro
2 mg/kg
Q3W
KEYNOTE-001: Adaptative design


