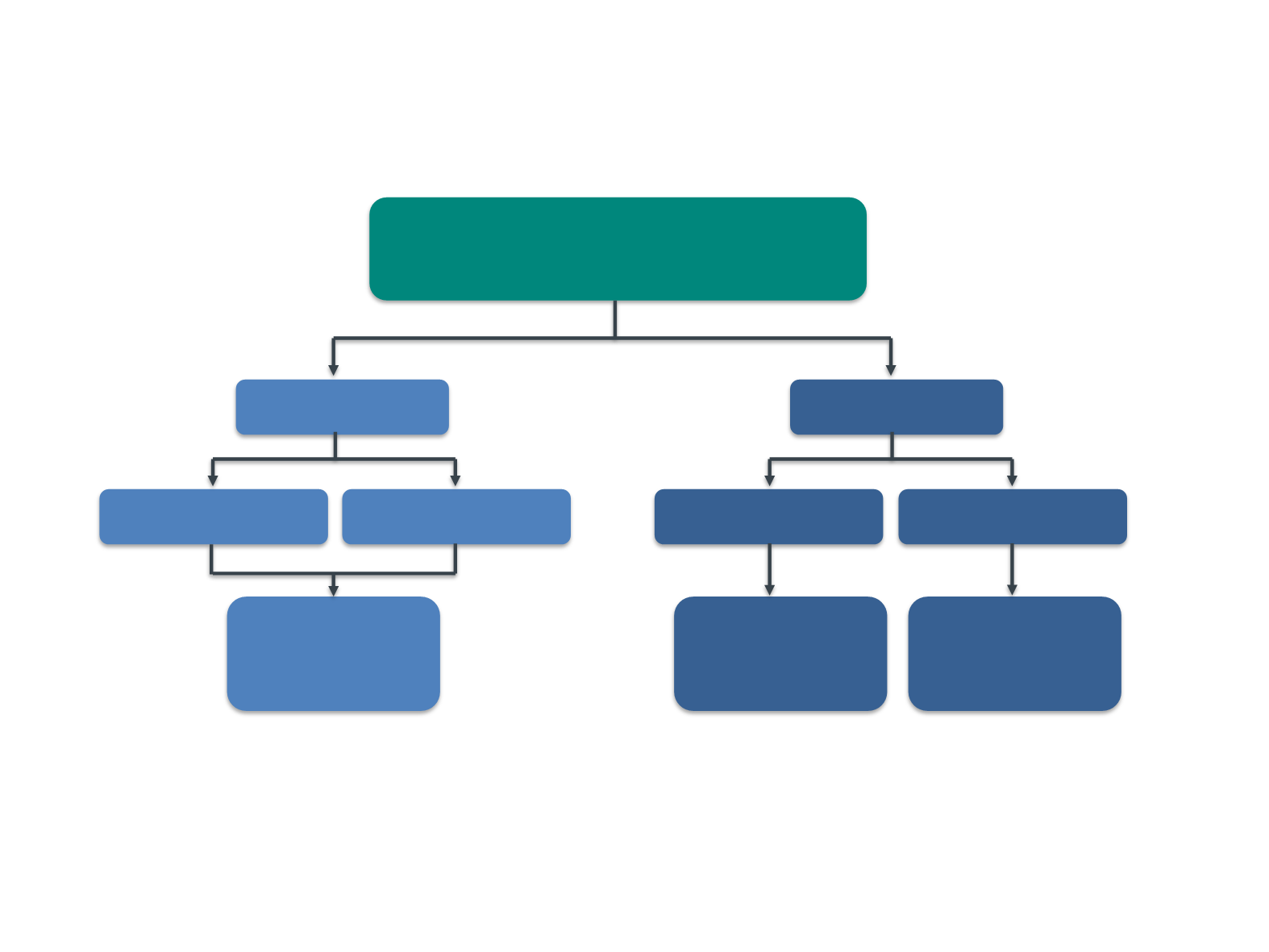
KEYNOTE-001 Trial:
Validation of PD-L1 Expression as a Biomarker
NSCLC = non–small cell lung cancer; PD-L1 = programmed death ligand 1; TPS = tumor proportion score.
1.
Garon EB et al.
N Engl J Med
. 2015;372(21):2018–2028.
Assignment to Training and Validation Groups in KEYNOTE-001
1
Patients who received ≥1 dose of KEYTRUDA
®
(pembrolizumab)
N=495
Training set
N=182
Validation set
N=313
Previously treated
n=171
Treatment naïve
n=11
Previously treated
n=223
Treatment naïve
n=90
Cut point selection
population
n=129
Biomarker
evaluable
population
n=156
Biomarker
evaluable
population
n=48
•
PD-L1 expression evaluation in the training group led to selection of the cut point
(TPS ≥50%)
1
•
Assessment in the validation group confirmed the cut point selection and value of
PD-L1 as a biomarker in NSCLC
1


