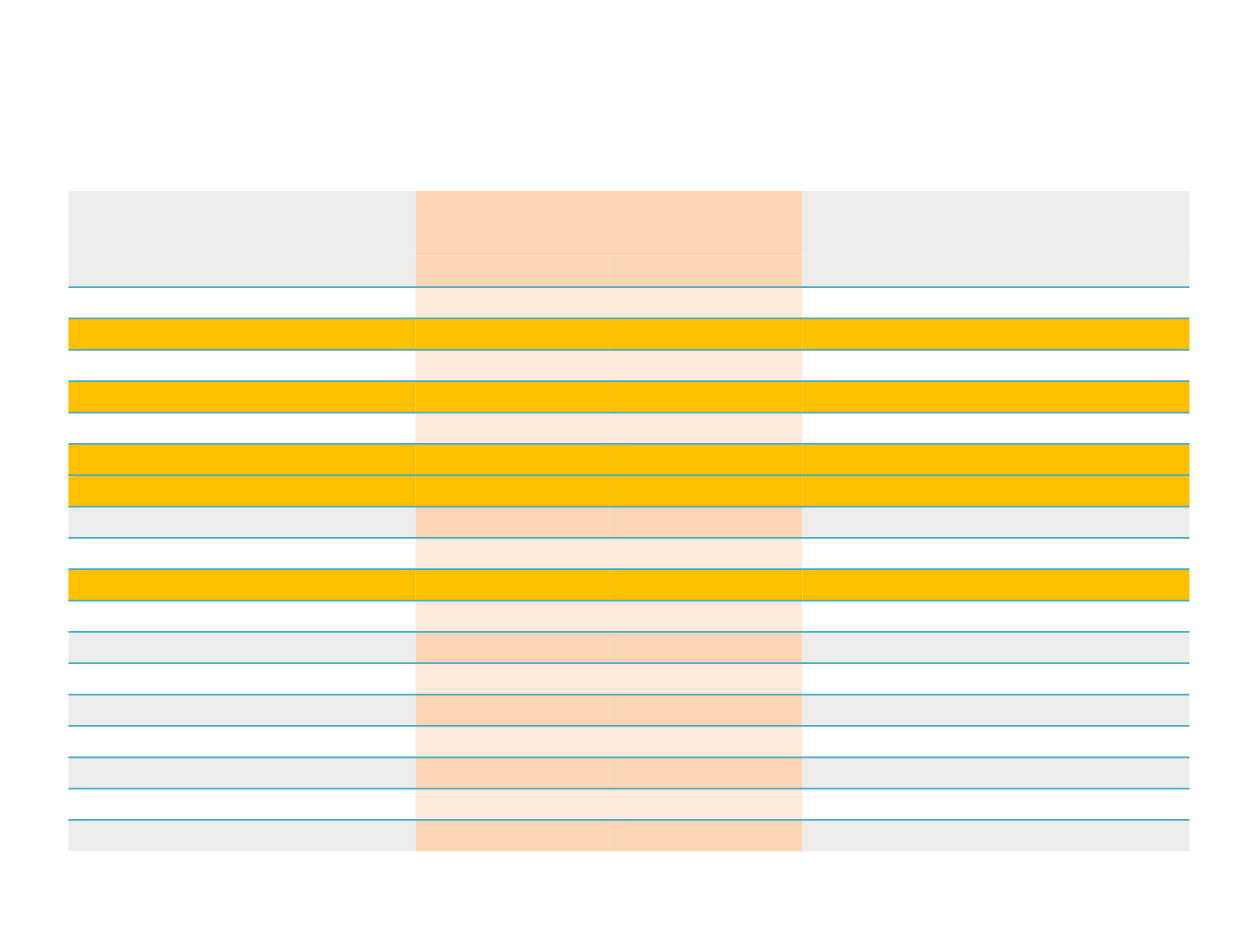
Adverse Events Occurring in ≥5% of the Patients
With Adenocarcinoma
Reck
et al
. Lancet Oncol. 2014;15:143-55. Suppl.
Nintedanib + docetaxel (n=320)
n (%)
Placebo + docetaxel (n=333)
n (%)
All grades
Grade ≥3
All grades
Grade ≥3
Any AE
308 (96.3)
243 (75.9)
314 (94.3)
228 (68.5)
Diarrhoea
139 (43.4)
20 (6.3)
82 (24.6)
12 (3.6)
Neutrophil count decreased
131 (40.9)
116 (36.3)
135 (40.5)
116 (34.8)
ALT increased
121 (37.8)
37 (11.6)
31 (9.3)
3 (0.9)
Fatigue
99 (30.9)
15 (4.7)
98 (29.4)
14 (4.2)
AST increased
97 (30.3)
13 (4.1)
24 (7.2)
2 (0.6)
Nausea
91 (28.4)
3 (0.9)
59 (17.7)
2 (0.6)
White blood cell count decreased
89 (27.8)
63 (19.7)
94 (28.2)
61 (18.3)
Decreased appetite
75 (23.4)
4 (1.3)
52 (15.6)
5 (1.5)
Vomiting
62 (19.4)
4 (1.3)
41 (12.3)
2 (0.6)
Alopecia
56 (17.5)
1 (0.3)
68 (20.4)
0 (0)
Dyspnoea
54 (16.9)
15 (4.7)
52 (15.6)
20 (6.0)
Neutropenia
44 (13.8)
38 (11.9)
51 (15.3)
45 (13.5)
Cough
42 (13.1)
3 (0.9)
63 (18.9)
2 (0.6)
Pyrexia
39 (12.2)
2 (0.6)
47 (14.1)
1 (0.3)
Stomatitis
36 (11.3)
4 (1.3)
26 (7.8)
1 (0.3)
Haemoglobin decreased
35 (10.9)
3 (0.9)
46 (13.8)
7 (2.1)
Constipation
22 (6.9)
0 (0)
39 (11.7)
1 (0.3)
Adverse events were classified according to Common Terminology Criteria for Adverse Events version 3.0
LUME-Lung


