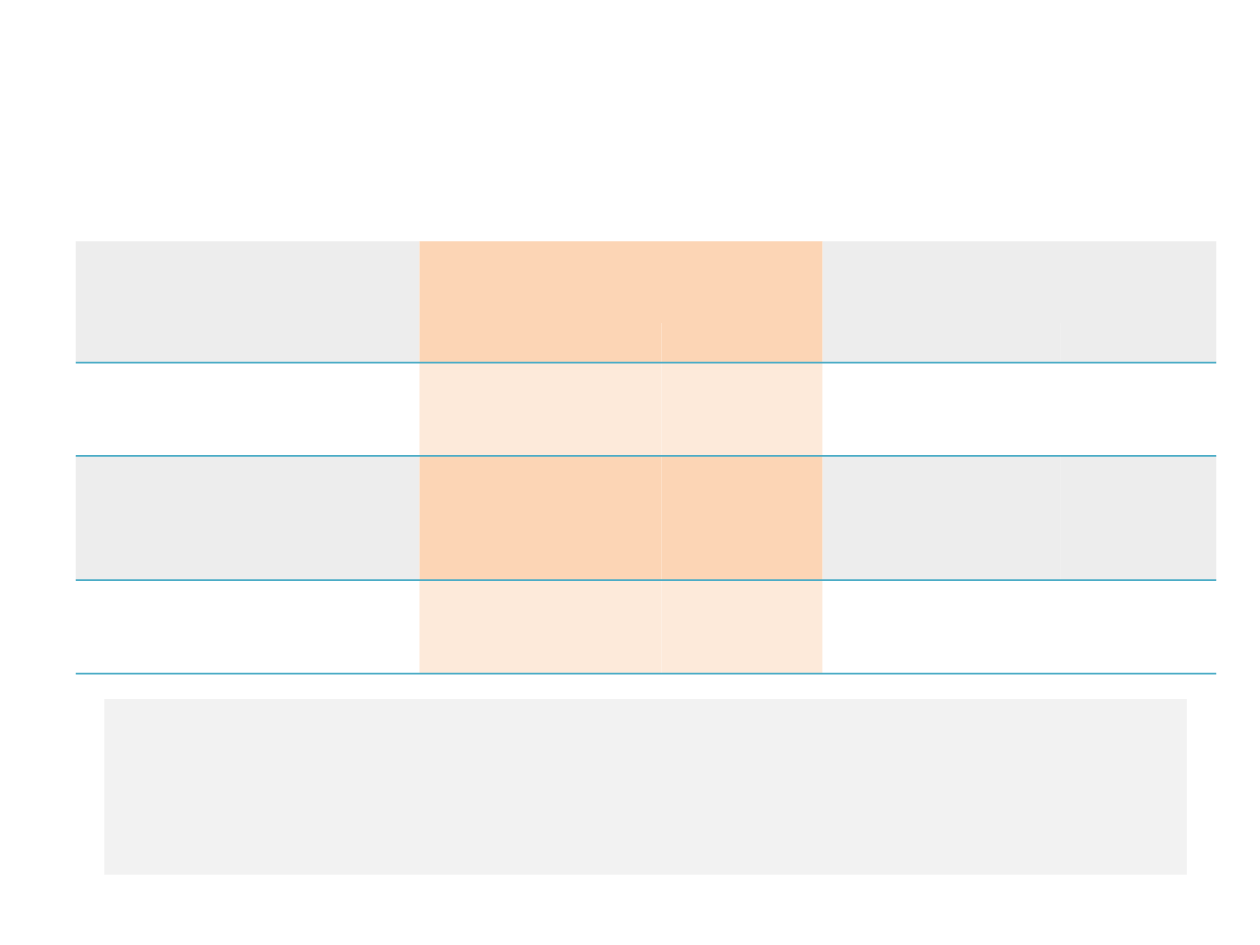
Adverse Events Leading to Discontinuation and
Dose Reduction (Adenocarcinoma Population)
Reck
et al
. Lancet Oncol. 2014;15:143-55. Suppl.
Nintedanib + docetaxel (n=320)
n; %
Placebo + docetaxel (n=333)
n; %
All grades
Grade ≥3
All grades
Grade ≥3
Any AE leading to
permanent
discontinuation
of last study
medication
67; 20.9%
57; 17.8%
59; 17.7%
43; 12.9%
Any AE leading to
dose reduction of nintedanib/
placebo
69; 21.6%
(17%, 1 dose reduction)
(4,6%, 2 dose reduction)
41; 12.8%
22; 6.6%
(6.6%, 1 dose reduction)
19; 5.7%
Any AE leading to
dose reduction of docetaxel
53; 16.6%
41; 12.8%
41; 12.3%
32; 9.6%
LUME-Lung
•
The median number of
Docetaxel courses:
4
(1-45) in the nintedanib arm
vs.
4
(1-42) placebo
The mean
dose intensity of Docetaxel
was
>98%
in both arms
•
The mean
dose intensity of Nintedanib
was
92%
and Placebo 94%
•
90 pts (
28%
) of pts continued with Nintedanib as monotherapy


