-100
-80
-60
-40
-20
0
20
40
60
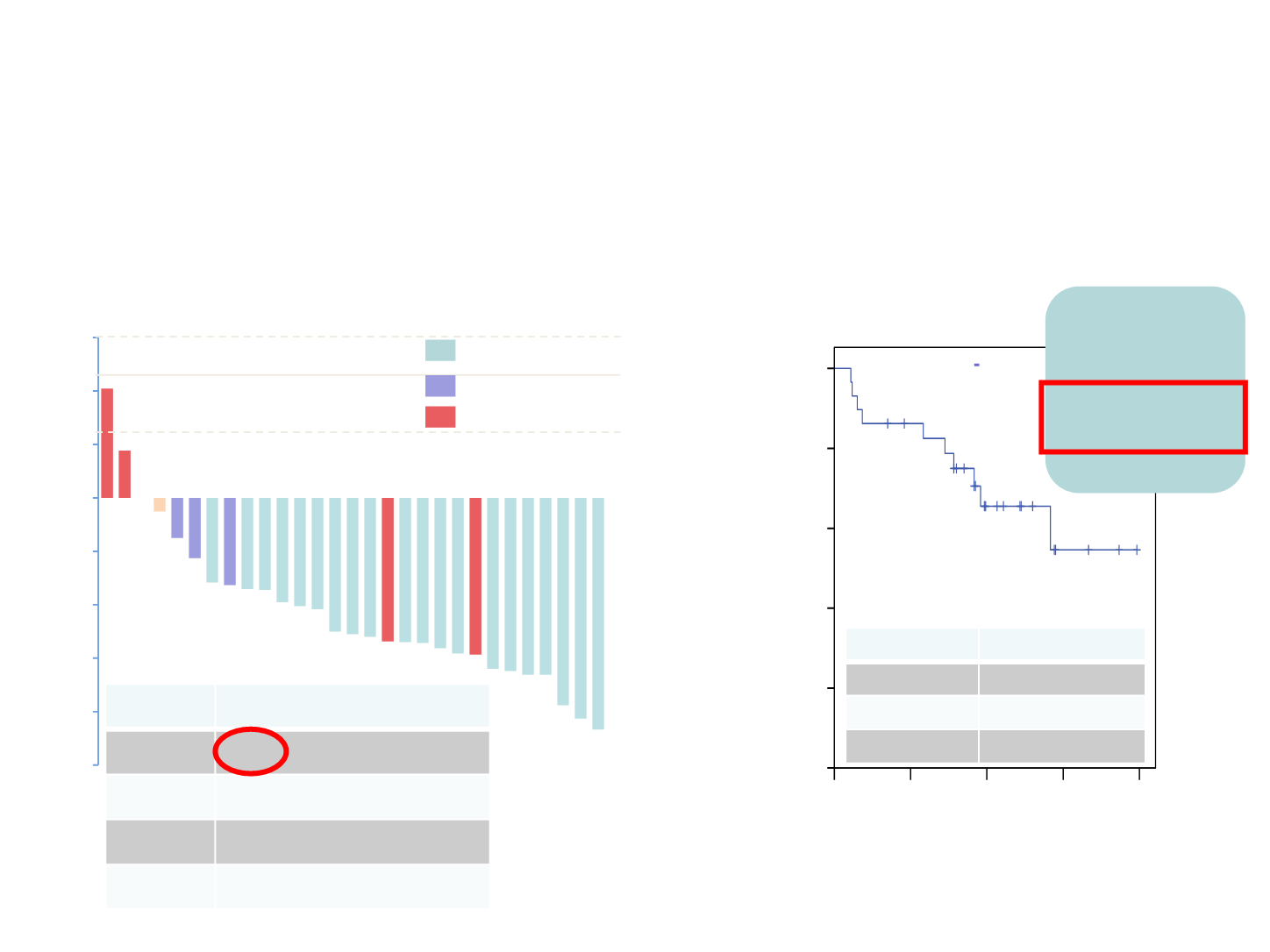
Partial response (PR)
Stable disease (SD)
Progressive disease (PD)
(N=30) % (N; 95% CI)
ORR
66.7 (20; 47.1-82.1)
DCR
83.3 (25; 64.6-93.7)
PD
13.3 (4; 4.4-31.6)
NE
3.3 (1; 0.2-19.1)
100
80
60
40
20
0
0
6
12
18
24
Progression-free survival (%)
Time (months)
(N=30)
months (95% CI)
Med. PFS
NR
Longest PFS 23.8
Med. FU
13.3 (9.6-17.0)
*Investigator assessed
Michels S, et al. WCLC 2016
EUCROSS
A European Phase II Trial of Crizotinib in Advanced Adenocarcinoma of the Lung
Harboring ROS1 Rearrangements - Preliminary Results
•
Locally
advanced/
metastasized
adNSCLC
•
Treatment naïve
or pre-treated
•
ECOG 0-2
•
≥ 18 years


