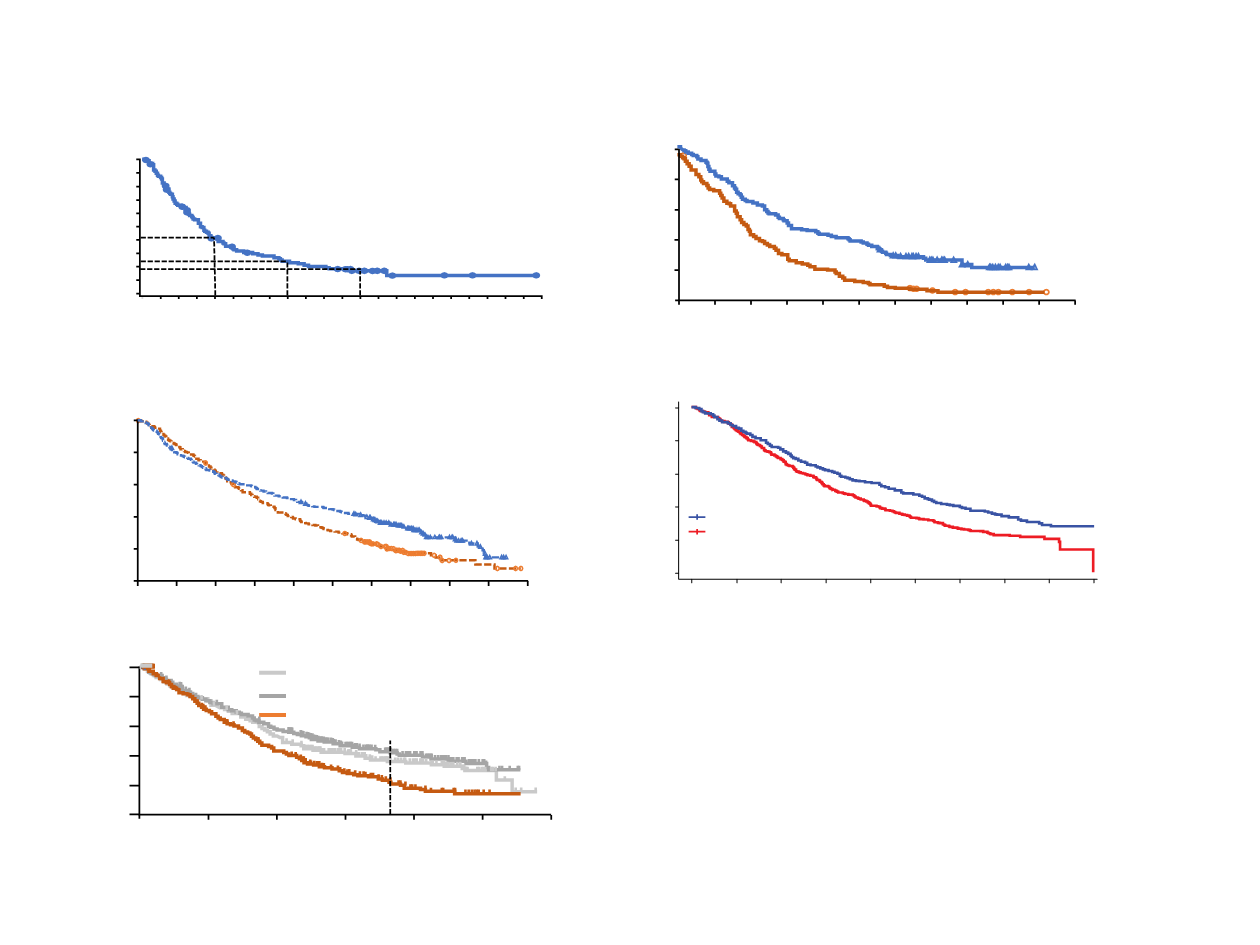
Long term-survival in 2L NSCLC
.
Gettinger SN. J Clin Oncol. 2015;33(18):2004-2012. 2. Borghaei H et al. Poster presentation at ASCO 2016. 9025. 3. Barlesi F et al. Oral
.
CA209-003: Nivo Monotherapy in NSCLC
1
Time Since First Dose (months)
100
80
60
0
40
20
0 6 12 18
30
24
36 42 48 54
66
60
3-year OS=18%
2-year OS=24%
OS (%)
1-year OS=42%
Checkmate 017: Squamous NSCLC
2
OS (%)
100
80
60
40
0
20
33
27
24
21
18
15
12
9
6
3
0
30
Docetaxel
2-yr OS =23%
2-yr OS=8%
Nivolumab
Time (months)
Checkmate 057: Non-squamous NSCLC
2
Time (months)
27
18
15
9
6
21
12
3
0
24
30
100
80
60
40
0
20
OS(%)
Docetaxel
2-yr OS=29%
2-yr OS=16%
Nivolumab
OS (%)
18-mo OS=40%
100
80
60
40
20
0
0
3
6
9 12 15 18 21 24 27
Atezolizumab
Docetaxel
18-mo OS=27%
Time (months)
OAK: NSCLC
3
18-mo OS=37%
18-mo OS=43%
18-mo OS=24%
KN-010: ≥1% PD-L1 NSCLC
4
Pembro 2 mg/kg
Docetaxel
Pembro 10 mg/kg
0
5
OS (%)
0
20
40
60
80
100
10
15
20
25
30


