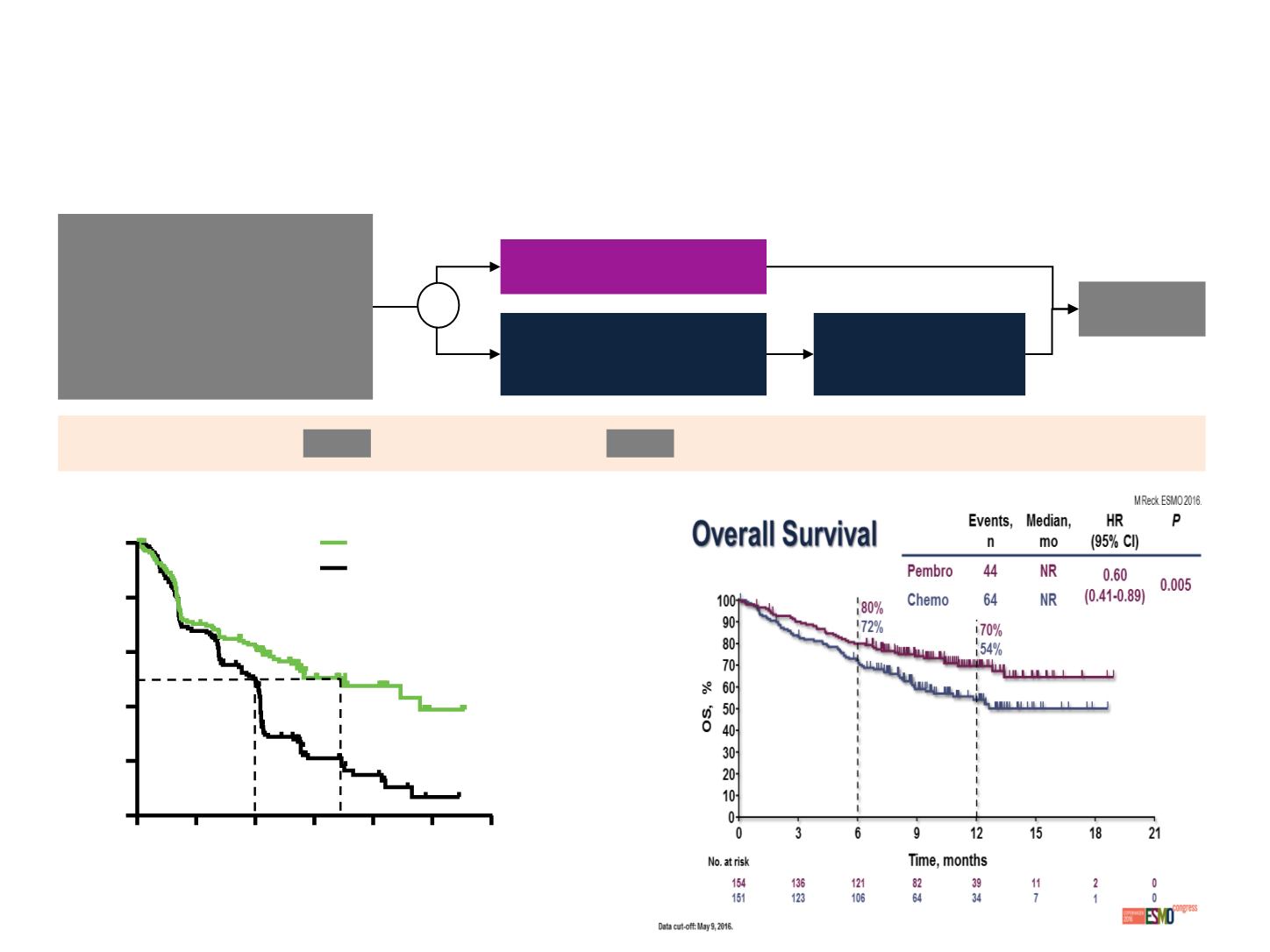
1L: PHASE III STUDY KEYNOTE-024
PEMBROLIZUMAB
VS
CT IN PD-L1 >50%
•
Reck, et al. ESMO 2016
•
Stage IV NSCLC (
EGFR
WT,
ALK
-)
•
Squamous or non-squamous
•
PD-L1 ≥50% by IHC
•
No prior chemotherapy
•
ECOG PS 0–1
(n=305)
Paclitaxel or pemetrexed or
gemcitabine + carboplatin or cisplatin
(4–6 cycles)
1
2
PFS
OS, ORR, PFS in patients with any PD-L1 status
Endpoints
Pembrolizumab 200mg i.v. q3w (max.
35 cycles)
Optional pemetrexed
maintenance
Until PD
Crossover to
pembrolizumab permitted
R
1.0
0.8
0.6
0.4
0.2
0
0
Time (months)
PFS estimate
6
3
9
18
15
12
Pembrolizumab
Chemotherapy
HR=0.50
(95% Cl 0.37–0.68)
p<0.001
PFS
6.0
10.3


