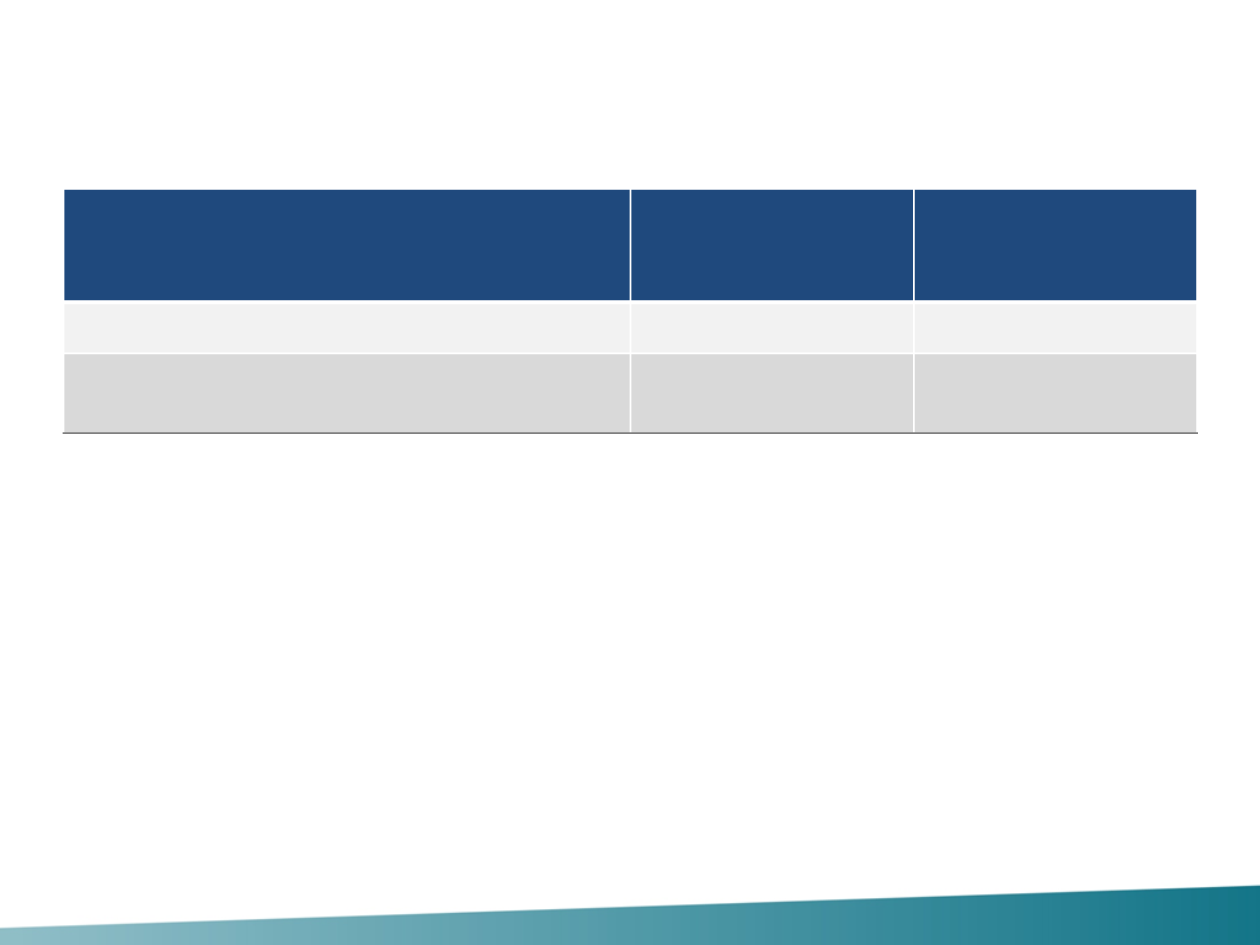
Cardiac Safety
* In patients with post-baseline assessment; n = 378 in the placebo group and 394 in the pertuzumab group.
sLVD, symptomatic left ventricular dysfunction.
•
One new sLVD event in the pertuzumab group after
40 months (resolved)
•
LVEF declines reversed in 88% of pertuzumab patients
Safety population
Placebo
+ T + D
(n = 396), %
Pertuzumab
+ T + D
(n = 408), %
sLVD
1.8
1.5
LVEF decline to < 50% and by ≥ 10%
points from baseline*
7.4
6.1
Swain S, NEJM 2015


