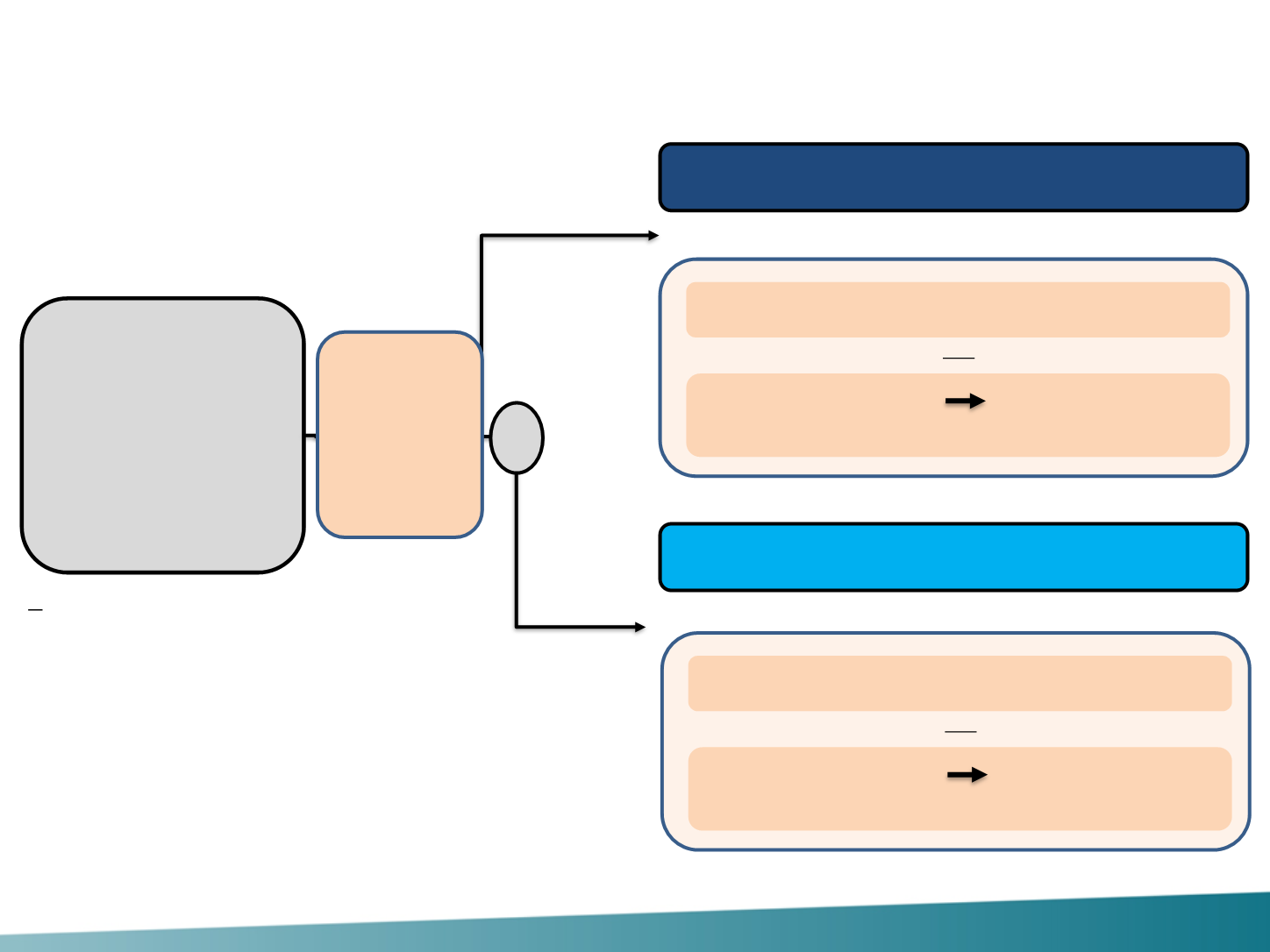
PERTAIN Study Design (Phase II Trial)
Postmenopausal
patients with HER2-
positive and hormone
receptor-positive
LA/MBC, not previously
treated with systemic
non-hormonal
anticancer therapy in the
advanced setting
(N = 258)*
Stratification factors:
•
Chemotherapy (yes/no)
•
Time since adjuvant hormone therapy
(<12 months/≥12 months/no prior therapy)
Pertuzumab + Trastuzumab
Trastuzumab
* 165 events to detect significant improvement in PFS from 7 months to 10.8 months (i.e. HR 0.645) with 80% power and a 2-sided log-rank test at an alpha level of 0.05.
†
Choice of chemotherapy must be specified before randomization; administered per product labelling. LA, locally advanced; R, randomization.
+
Aromatase Inhibitor
Docetaxel or Paclitaxel Aromatase Inhibitor
(18–24 weeks)
†
OR
+
Aromatase Inhibitor
Docetaxel or Paclitaxel Aromatase Inhibitor
(18–24 weeks)
†
OR
R
Choice of
chemotherapy
must be
specified before
randomization
Arpino G, SABCS 2016
> 6 m since adjuvant tx


