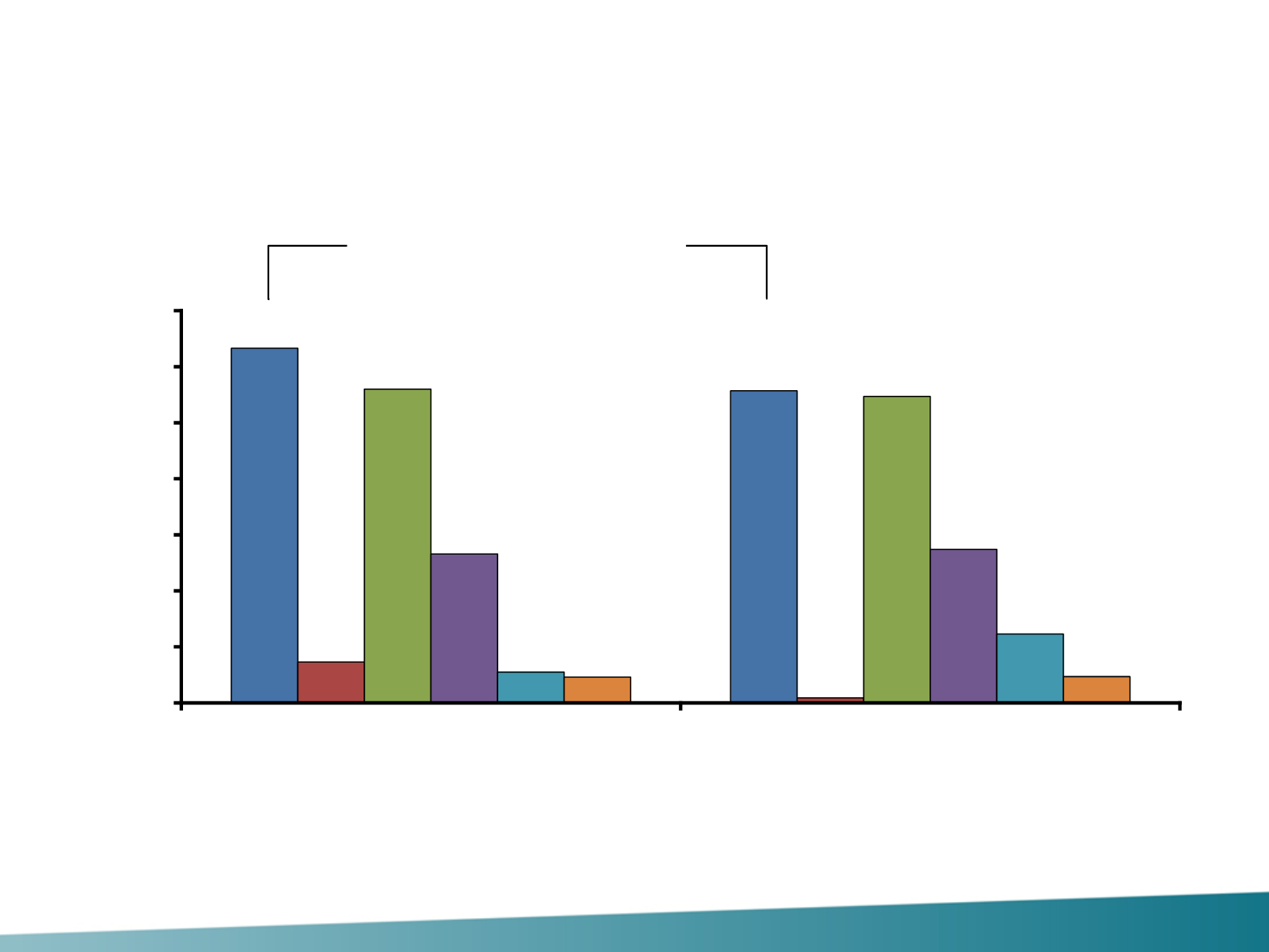
Overall Response Rate (ITT Population with Measurable
Disease at Baseline)
Based on best overall response according to RECIST Version 1.1. NE: Patients who did not have any evaluable post-baseline assessments.
95% CIs were computed using the Clopper
–
Pearson approach. 95% difference in ORR between treatment arms with associated 95% CIs calculated using the Hauck
–
Anderson approach.
CR, complete response; NE, not evaluable; PR, partial response; SD, stable disease.
63,3
55,7
7,3
0,9
56,0
54,7
26,6
27,4
5,5
12,3
4,6
4,7
0
10
20
30
40
50
60
70
Pertuzumab + Trastuzumab + AI
(n = 109)
Trastuzumab + AI
(n = 106)
Patients, %
Δ ORR = 7.6% (95% CI –6.0, 21.3)
Chi-squared p = 0.2537
ORR CR
PR
SD
PD
NE
ORR
CR
PR
SD
PD
NE


