Grade ≥3
Olaparib
(N=74)
Placebo
(N=62)
1 (1%)
0
5 (7%)
1 (2%)
2 (3%)
0
2 (3%)
1 (2%)
0
2 (3%)
4 (5%)
1 (2%)
0
0
0
0
0
0
BRCA
m (N=96)
Preferred term (%)
All grades
Olaparib
(N=74)
Placebo
(N=62)
Nausea
54 (73%)
20 (32%)
Fatigue
40 (54%)
23 (37%)
Vomiting
27 (36%)
5 (8%)
Diarrhoea
22 (30%)
12 (19%)
Abdominal pain
17 (23%)
18 (29%)
Anaemia
19 (26%)
3 (5%)
Constipation
14 (19%)
7 (11%)
Decreased appetite
14 (19%)
6 (10%)
Abdominal pain upper
14 (19%)
4 (6%)
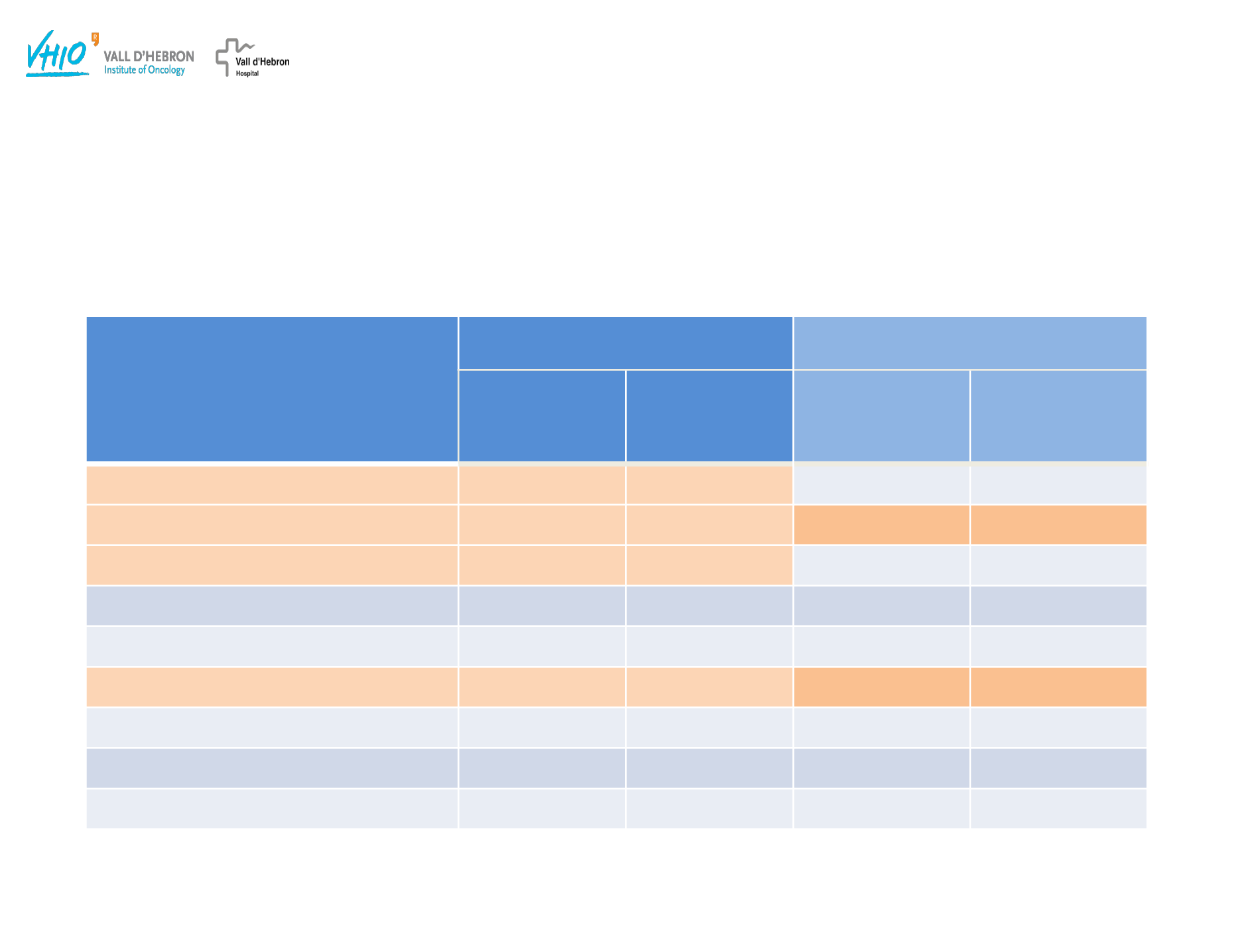
Ledermann J et al. Lancet Oncol 2014;15:852–861
Study 19: Toxicity Profile
The tolerability profile reported in patients with BRCA mutated was similar to the
overall population
Dose Interruption ( 36%) and Dose Reductions( 42%) in the Olaparib group
were most due to Nausea, Vomiting and Fatigue


