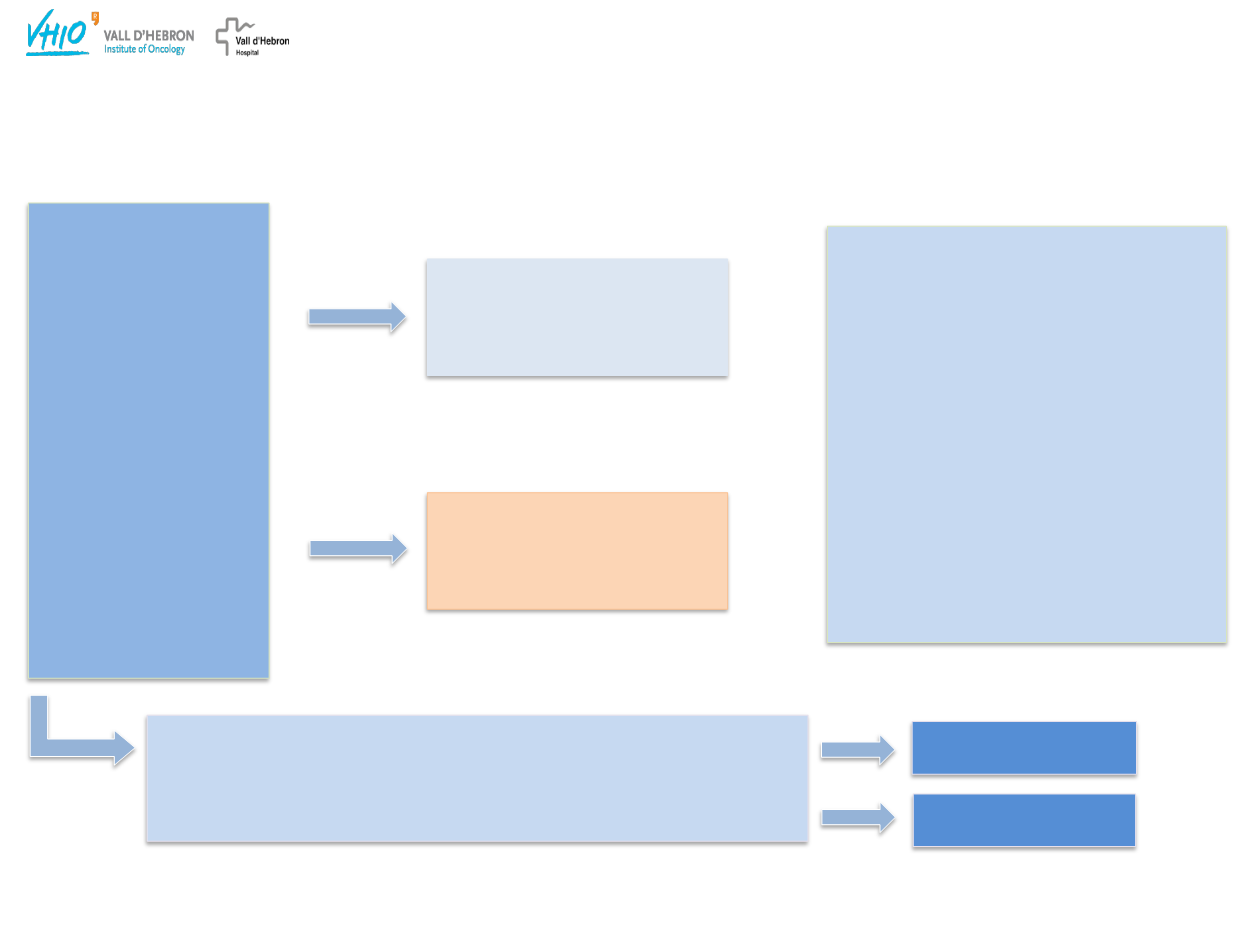
Study 19: Phase II trial design, endpoints and
BRCA
testing
N=265
•
‘Platinum-
sensitive’ recurrent
high-grade serous
ovarian cancer
•
≥2 prior regimens
of platinum-based
chemotherapy
•
Complete or partial
response to most
recent platinum-
based regimen
Olaparib maintenance
monotherapy
(400 mg bid, capsules)
n=136
n=129
Placebo
(bid, capsules)
Double-blind
randomization
1:1
Treatment until progression
BRCA
testing:
•
Previous local germline
BRCA
testing (case report forms)
•
Retrospective germline
BRCA
testing or tumour
BRCA
testing
BRCA
m: n=136
BRCA
wt n=118
Primary endpoint:
Progression-free survival
(PFS)
by RECIST 1.0
Secondary endpoints included:
Overall survival
(OS),
safety and tolerability
Exploratory endpoints:
Time to first subsequent therapy
or death
(TFST),
time to second
subsequent therapy or death
(TSST)
Ledermann J
et al
.
N Engl J Med
2012;366:1382–1392
OLAPARIB :A 10 year development journey
Randomized Clinical Trials : Phase II/III


