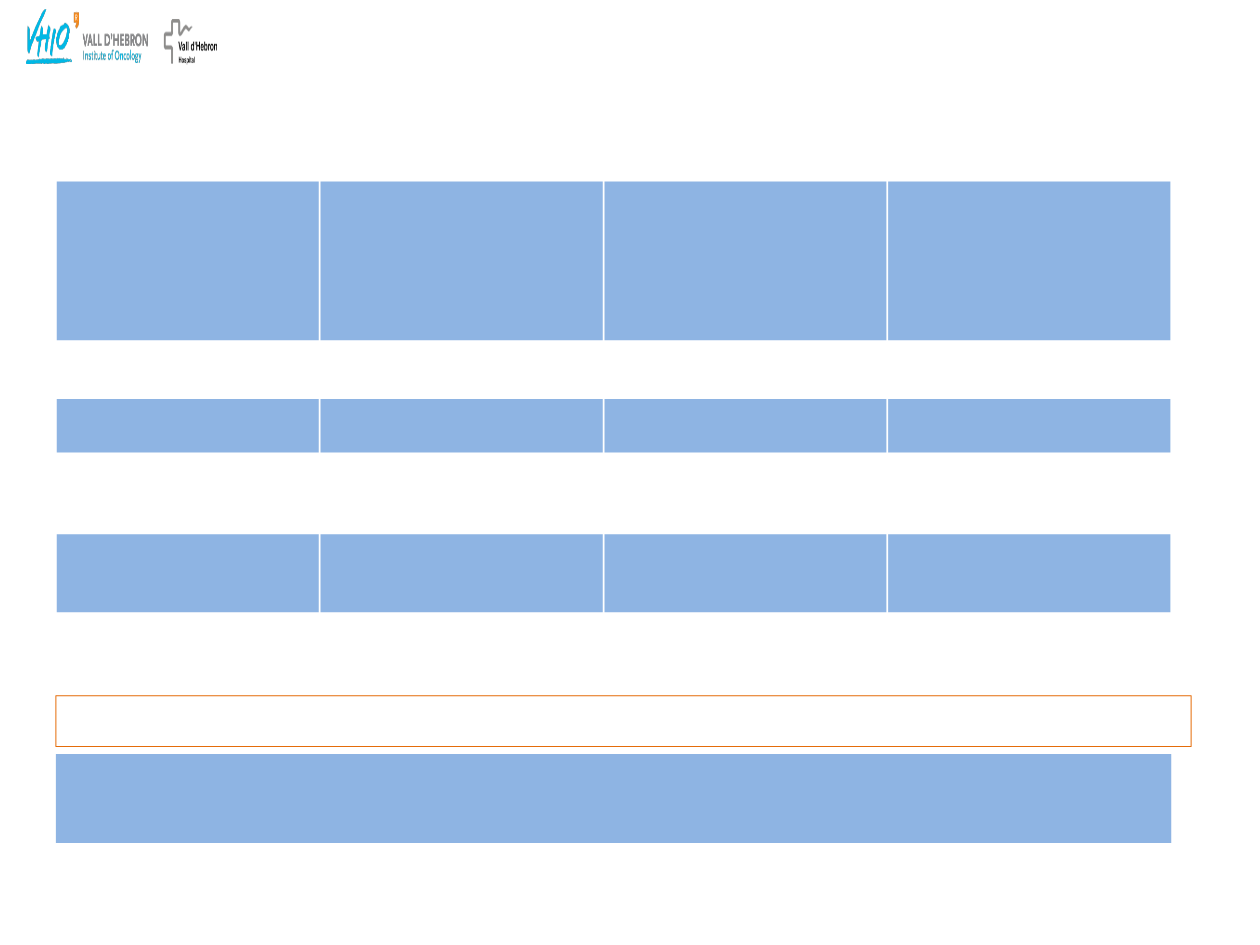
Olaparib
Phase I and
BRCA
mutation expansion
studies in ovarian cancer
patients
1
Olaparib multicenter
Phase II
BRCA
mutation
ovarian cancer study
2
Olaparib multicenter
Phase II
BRCA
+/– study
(ovarian cancer
patients)
3
Olaparib patients
n=50
n=33
n=64
Olaparib dose
200 mg bid
400 mg bid
400 mg bid
RECIST response (CR
+ PR)
28%
33%
BRCA
+ 41%
BRCA
– 24%
Disease control
rate
*
46%
69%
BRCA
+ 76%
BRCA
– 62%
Median duration of
response
7.0 months
9.5 months
Not reported
*
Complete response (CR) + partial response (PR) + stable disease (SD)
1.Fong PC
et al
.
J Clin Oncol
2010;28:2512–2519;
2. Audeh MW
et al
.
Lancet
2010;376:245–251;
3. Gelmon KA
et al
.
Lancet Oncol
2011;12:852–861
Provides clinical evidence of activity in ovarian cancer patients with and
without
BRCA1/2
mutations
Early Clinical Trials(3):
Olaparib( AZD2281): an orally active PARP inhibitor in ovarian cancer


