Ongoing phase Ib/II study in postmenopausal patients (n=77)
with anastrozole- or letrozole-resistant HR+/HER2– MBC
RP2D: ribociclib 300 mg/day (3 weeks on and 1 week off) everolimus
2.5 mg/day (continuous) and exemestane 25 mg/day (continuous)
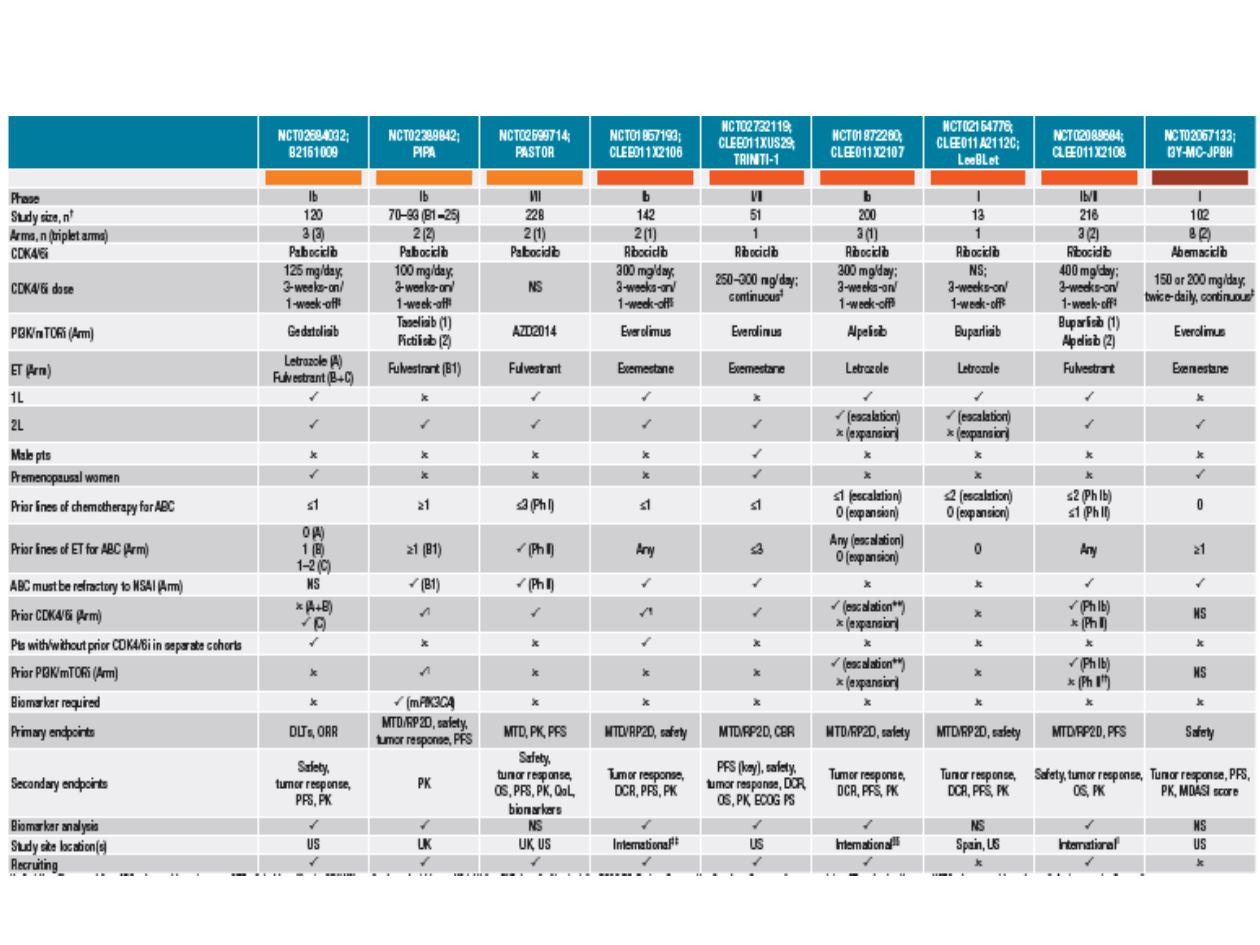
CDK4/6 i + PI3K/mTOR i + AIs: ongoing trials
R2PD, recommended phase 2 dose
Cortes et al. CoBrCa 2016
.
Overall response rate:
9% (95% CI 4–18%)
Clinical benefit rate:
26% (95% CI 16–37%)
Most common drug-related grade 3/4 AEs:
•
Neutropenia (31%)
•
Neutrophil count reduction (18%)
•
Leukopenia (12%)
Triplet combination with ribociclib-EVE-EXE had a manageable safety profile with
encouraging signs of clinical activity in a heavily pretreated patient population


