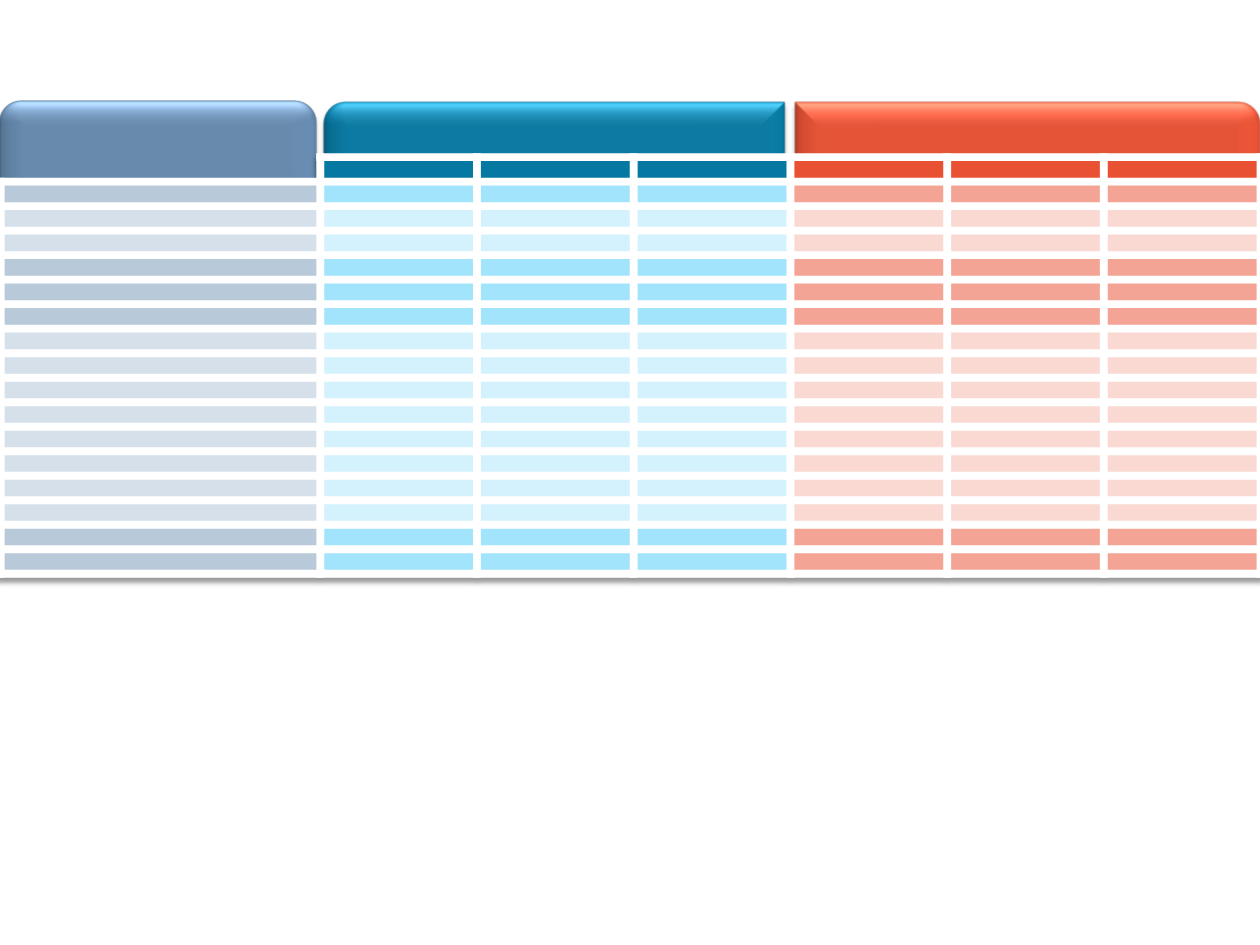
Phase III MONALEESA-2
In the ribociclib arm 10 (3.0%) patients experienced Grade 2 QTcF (481–500 ms) and 1
(0.3%) patient experienced Grade 3 QTcF (>500 ms); no dose reductions were required
Adverse Event
≥15% in Either Arm, %
Ribociclib + Letrozole
n=334
Placebo + Letrozole
n=330
All
Grade 3
Grade 4
All
Grade 3
Grade 4
Nausea
52
2.4
0
29
0.6
0
Infections
50
3.6
0.6
42
2.1
0.3
Fatigue
37
2.1
0.3
30
0.9
0
Diarrhea
35
1.2
0
22
0.9
0
Alopecia
33
–
–
16
–
–
Vomiting
29
3.6
0
16
0.9
0
Arthralgia
27
0.6
0.3
29
0.9
0
Constipation
25
1.2
0
19
0
0
Headache
22
0.3
0
19
0.3
0
Hot flush
21
0.3
0
24
0
0
Back pain
20
2.1
0
18
0.3
0
Cough
20
0
–
18
0
–
Decreased appetite
19
1.5
0
15
0.3
0
Rash
17
0.6
0
7.9
0
0
ALT increased
16
7.5
1.8
3.9
1.2
0
AST increased
15
4.8
0.9
3.6
1.2
0
Hortobagyi G, et al. NEJM 2016


